Improved preparation method for lacosamide intermediate
A technology of benzylamine and reaction time, applied in the field of preparation of 2-(1-butyric acid-4-yl)-amino-substituted butanamide, can solve the problem of reduced yield, product racemization, and lack of effective removal methods and other problems, to achieve the effect of easy temperature and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
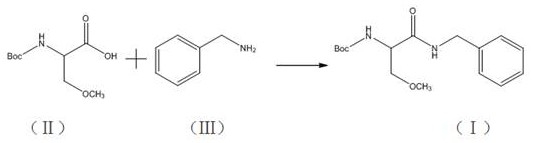
[0026] Weigh 50.0 g of N-(tert-butoxycarbonyl)-O-methylserine and 500 ml of dichloromethane into a three-neck flask, cool down in an ice-water bath, and stir. 27.2 g of ethyl chloroformate and 25 ml of 2,6-di-tert-butylpyridine were added dropwise in sequence. After diluting 25.0 g of benzylamine with 50 ml of dichloromethane, it was slowly added dropwise to the system in an ice-water bath. React at 0-10°C for 1 hour and 40 minutes. Stop the reaction, add 500 ml of water solution, and collect the organic phase. Add 125 ml of 3N hydrochloric acid to the organic phase, separate the layers, and collect the organic phase. Add 200 ml of saturated sodium bicarbonate solution to the organic phase, separate the layers, and collect the organic phase. Add 500 ml of water to wash the organic phase, and separate the layers. After the organic phase was collected, the solvent was spinned off, 350 ml of petroleum ether was added, and the temperature was crystallized in an ice-water bath....
Embodiment 2
[0028] Weigh 100.0 g of N-(tert-butoxycarbonyl)-O-methylserine and 1000 ml of dichloromethane into a three-neck flask, cool down in an ice-water bath, and stir. 54.4 g of ethyl chloroformate and 50 ml of 2,6-di-tert-butylpyridine were added dropwise in sequence. After diluting 50.0 g of benzylamine with 100 ml of dichloromethane, it was slowly added dropwise to the system in an ice-water bath. React at 0-10°C for 2 hours and 10 minutes. Stop the reaction, add 1000 ml water solution, and collect the organic phase. Add 250 ml of 3N hydrochloric acid to the organic phase, separate the layers, and collect the organic phase. Add 400 ml of saturated sodium bicarbonate solution to the organic phase, separate the layers, and collect the organic phase. Add 1000 ml of water to wash the organic phase, and separate the layers. After the organic phase was collected, the solvent was spun off, 700 ml of petroleum ether was added, and the temperature was cooled in an ice-water bath for cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com