Method for testing sealing integrity of medicine packaging container by improved color water intrusion method
A packaging container and water intrusion technology, which is applied in fluid tightness testing, liquid tightness measurement using liquid/vacuum degree, machine/structural component testing, etc. It can solve the problem that the sensitivity cannot continue to meet the requirements and the anti-interference ability is poor , interference detection and other issues, to achieve the effect of shortening the experimental cycle, strong anti-interference ability, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] instrument
[0029] Sealing tester, MFY-05S, Jinan Sanquan Zhongshi Experimental Instrument Co., Ltd.
[0030] Fluorescence spectrophotometer, F-2710, Hitachi (China) Co., Ltd.
[0031] Electronic balance, XS205DU, METTLER TOLEDO
[0032] Ultrapure Water Meter, Milli-Q Reference, Millipore
[0033] materials
[0034] Sodium fluorescein, P1605648, Adamas-beta
[0035] Sodium Hydroxide, C10921846, 96%, MACKLIN
[0036] Capillary, Shanghai Zhonglin Electromechanical Equipment Co., Ltd.
[0037] Steps
[0038] positive sample preparation
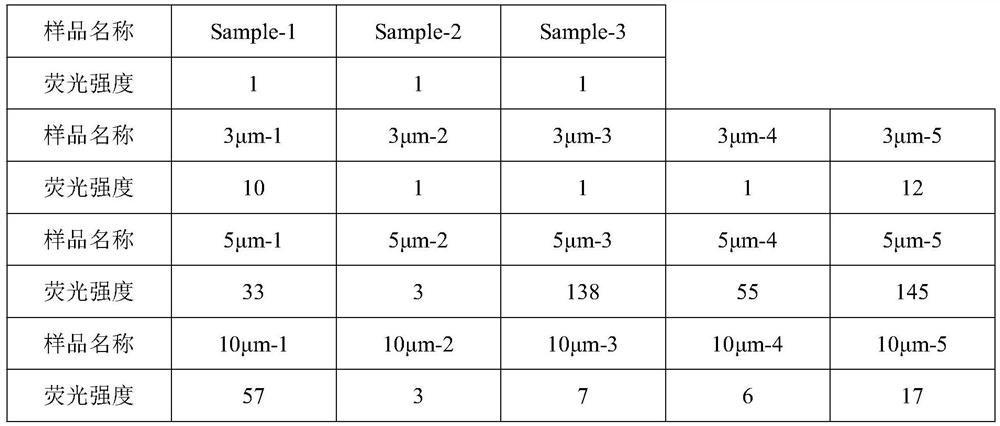
[0039] Take 15 sterile powders for injection (12mL specification) and divide them into a 3 μm positive sample group (5), a 5 μm positive sample group (5), and a 10 μm positive sample group (5). Remove the labels and plastic covers from the above samples, and clean the outer surface with ultrapure water. Use a capillary with a corresponding pore size to penetrate the rubber stopper through a stainless steel needle, pull out the ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com