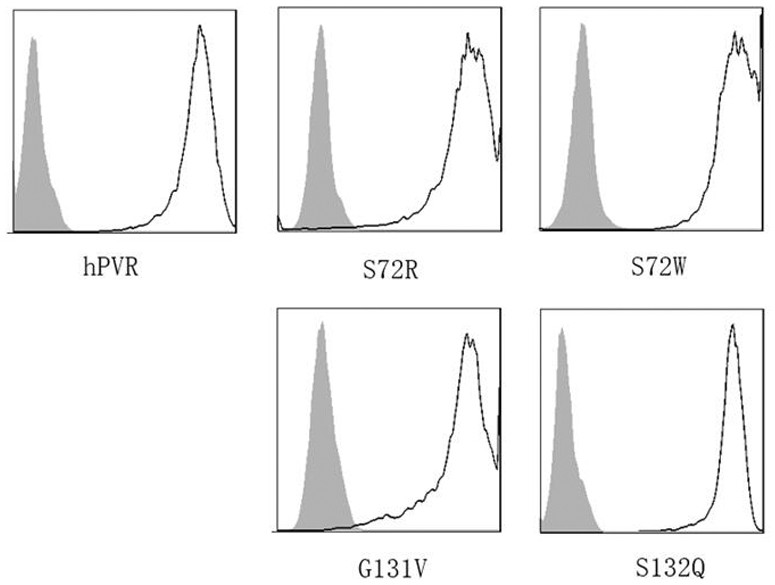
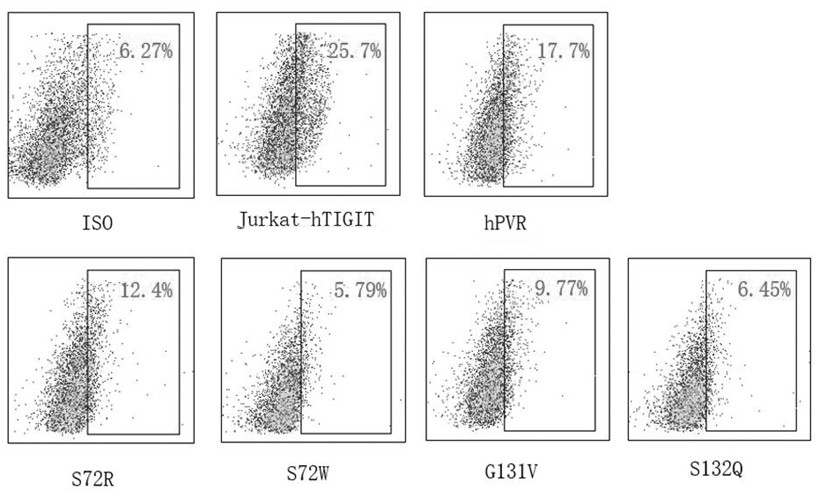
high affinity pvr mutant
A mutant and protein mutant technology, applied in the field of high-affinity PVR protein mutants, can solve the problems of tumor immune tolerance and immune escape, poor tumor treatment effect, T cell exhaustion, etc. Cytokine secretion, lowering the cost of treatment, lowering the effect of dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of lentiviral vector pLVXpuro-hPVR.
[0029] (1) PCR amplification: hPVR gene was amplified from cDNA by using KOD-Plus-Neo high-fidelity amplification enzyme (TOYOBO).
[0030] The amplification primers are:
[0031] hPVR-F:aaggatccgccaccatggcccgagc;
[0032] hPVR-R: cctctagatcaattacggcagctct;
[0033] The PCR conditions are: 94°C for 2min, 98°C for 10s, 55°C for 2min, and after 25 cycles, 68°C for 5min.
[0034] (2) PCR product gel recovery: After the PCR product was subjected to DNA gel electrophoresis, it was purified and recovered according to the instructions of the DNA gel recovery kit (AXYGEN) to obtain the hPVR gene fragment.
[0035] (3) Digestion of the target gene and vector: use endonuclease BamHI, XbaI (NEB), the target fragment recovered from the glue in step (2) and the lentiviral vector pLVX-puro (preserved by the laboratory) 37 Digestion at ℃, 2h.
[0036] (3) Gel recovery of digested products: After DNA gel electrophoresis...
Embodiment 2
[0039] Example 2: Construction of pLVXpuro-hPVR mutant protein lentiviral vector
[0040] (1) Design of PCR primers: According to the gene sequence and codon table of hPVR mutant proteins, primers for the four hPVR protein mutants were designed. The gene sequence of the mutant protein is thus as follows:
[0041] Mutant S72R:
[0042]
[0043]
[0044] Mutant S72W:
[0045]
[0046] Mutant G131V:
[0047]
[0048]
[0049] Mutant S132Q:
[0050]
[0051] The primer sequences are:
[0052] mutant S72R
[0053] hPVR-S72R-F: gcggcatggtgaacgtggcagcatggccgt;
[0054] hPVR-S72R-R:acggccatgctgccacgttcaccatgccgc;
[0055] mutant S72W
[0056] hPVR-S72W-F: gcggcatggtgaatggggcagcatggccgt;
[0057] hPVR-S72W-R:acggccatgctgccccattcaccatgccgc;
[0058] Mutant G131V
[0059] hPVR-G131V-F: cacgttcccgcaggtcagcaggagcgtgga;
[0060] hPVR-G131V-R:tccacgctcctgctgacctgcgggaacgtg;
[0061] mutant S132Q
[0062] hPVR-S132Q-F:gttcccgcagggccagaggagcgtggatat;
[0063] h...
Embodiment 3
[0071] Example 3: Construction of hPVR and hPVR gene mutant overexpression stable cell lines
[0072] 3.1 Cell Recovery and Culture
[0073] (1) Recovery of HEK-293T cells and CHO-K1 cells: Preheat fresh DMEM and RPMI 1640 medium in a 37°C water bath. Take out HEK-293T cells and CHO-K1 cells from the -80°C refrigerator, quickly put them into a 37°C water bath, and shake slowly to melt them. The thawed cell suspension was centrifuged at 1000 rpm at 25°C for 5 min. After centrifugation, the supernatant was discarded, and HEK-293T cells were added to 1 mL of preheated DMEM medium, and CHO-K1 cells were added to 1 mL of preheated RPMI 1640 medium to resuspend. Transfer the cell suspension to a petri dish, add the corresponding culture medium to 10mL, shake gently and place at 37°C, 5% CO 2 cultured in an incubator.
[0074] (2) Cell passage: When the cells cover the bottom of the dish, discard the old culture medium. Slowly add 10mL of PBS7.2 to wash the cells with a pipette ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com