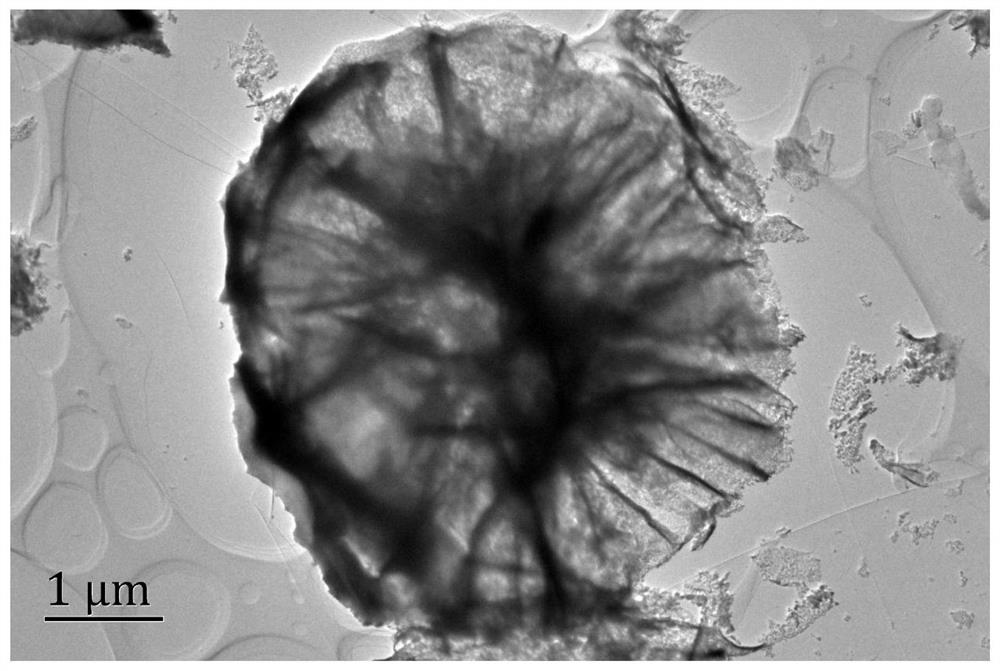
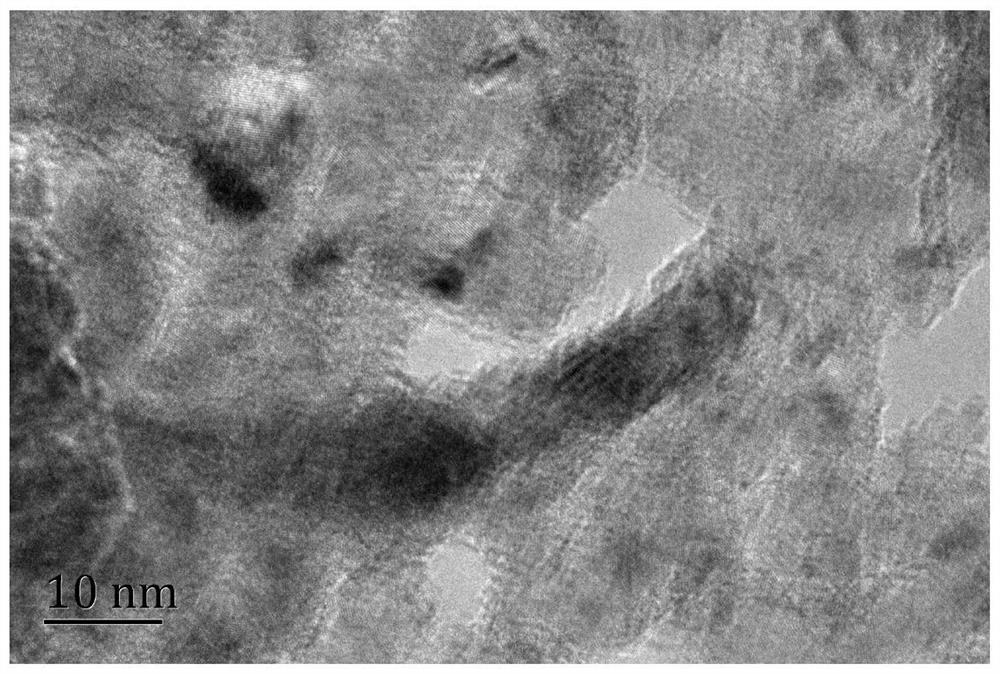
A kind of alkaline solution hydrogen evolution electrocatalyst nivru ternary alloy and its preparation method and application
A technology of alkaline solutions and ternary alloys, applied in electrodes, electrolytic components, electrolytic processes, etc., to achieve good electrochemical stability, short process, and excellent alkaline hydrogen evolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Weigh 2.7mmol nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O), 0.3mmol vanadium chloride (VCl 3 ) was dissolved in 60mL of purified water, and then 15mmol of urea (CH 4 N 2 O), stirred for 15 minutes to obtain a green mixed solution.
[0039] (2) Pour the above green mixed solution into a polytetrafluoroethylene hydrothermal reaction kettle, put the hydrothermal reaction kettle into a blast drying oven, react for 4 hours under hydrothermal conditions at 120°C, and turn off the blast drying Cool the reactor to room temperature;
[0040] (3) Centrifuge the product obtained in the reaction kettle, wash twice with pure water solvent, then wash twice with ethanol solvent, and put it into a vacuum drying oven to dry to obtain a light yellow powder;
[0041] (4) Weigh 20 mg of the yellow powder obtained in step (3) and dissolve it in 25 mL of pure aqueous solution, add 0.5 mg of RuCl 3 Stir to obtain a mixed solution;
[0042] (5) Pour the mixed solution obtained in...
Embodiment 2
[0051] (1) Weigh 8mmol nickel chloride (NiCl 2 ), 0.4mmol of vanadium chloride was dissolved in 60mL of purified water, then 18mmol of urea was added, and stirred for 15 minutes to obtain a green mixed solution.
[0052] (2) Pour the above green mixed solution into a polytetrafluoroethylene hydrothermal reaction kettle, put the hydrothermal reaction kettle into a blast drying oven, react for 2 hours under hydrothermal conditions at 150°C, and turn off the blast drying Cool the reactor to room temperature;
[0053] (3) Centrifuge the product obtained in the reaction kettle, wash twice with pure water solvent, then wash twice with ethanol solvent, and put it into a vacuum drying oven to dry to obtain a light yellow powder;
[0054] (4) Weigh 10 mg of the yellow powder obtained in step (3) and dissolve it in 50 mL of pure aqueous solution, add 1.0 mg of RuCl 3 Stir to obtain a mixed solution;
[0055] (5) Pour the mixed solution obtained in step (4) into a hydrothermal reactio...
Embodiment 3
[0060] (1) Weigh 12mmol of nickel sulfate and 3mmol of vanadium chloride in 80mL of pure water, then add 150mmol of urea and stir for 15 minutes to obtain a green mixed solution.
[0061] (2) Pour the above green mixed solution into a polytetrafluoroethylene hydrothermal reaction kettle, put the hydrothermal reaction kettle into a blast drying oven, react under hydrothermal conditions at 90°C for 10 hours, and turn off the blast drying Cool the reactor to room temperature;
[0062] (3) Centrifuge the product obtained in the reaction kettle, wash twice with pure water solvent, then wash twice with ethanol solvent, and put it into a vacuum drying oven to dry to obtain a light yellow powder;
[0063] (4) Weigh 20 mg of the yellow powder obtained in step (3) and dissolve it in 25 mL of pure aqueous solution, add 2.0 mg of RuCl 3 Stir to obtain a mixed solution;
[0064] (5) Pour the mixed solution obtained in step (4) into a hydrothermal reaction kettle, react under hydrothermal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com