Quantitative detection method of acryloyl chloride in ibrutinib bulk drug preparation process
A quantitative detection method and preparation process technology, which is applied in the field of quantitative detection of acryloyl chloride in the preparation process of ibrutinib bulk drug, can solve problems such as equipment damage and strong neurotoxicity, and achieve small instrument damage, strong specificity, and sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 1. Sample testing
[0079] Preparation of the test solution: Accurately measure 5mL of the reaction solution in the preparation process of the ibrutinib API with batch number 20201104, put it in a 10mL volumetric flask, add 0.1mL of the internal standard solution, and then use methanol, N,N-di The volume ratio of methylformamide and triethylamine is a mixed solvent of 98.5:0.5:1, dissolve and dilute to the mark, shake well, and set aside.
[0080] 2 parallel samples of the reference solution, 3 injections for the first sample, 2 injections for the second sample; 2 parallel samples for the test solution, 2 injections for the first sample, 2 injections for the second sample . Inject headspace and record chromatograms. The results showed that acryloyl chloride was not detected.
[0081] 2. Methodological verification
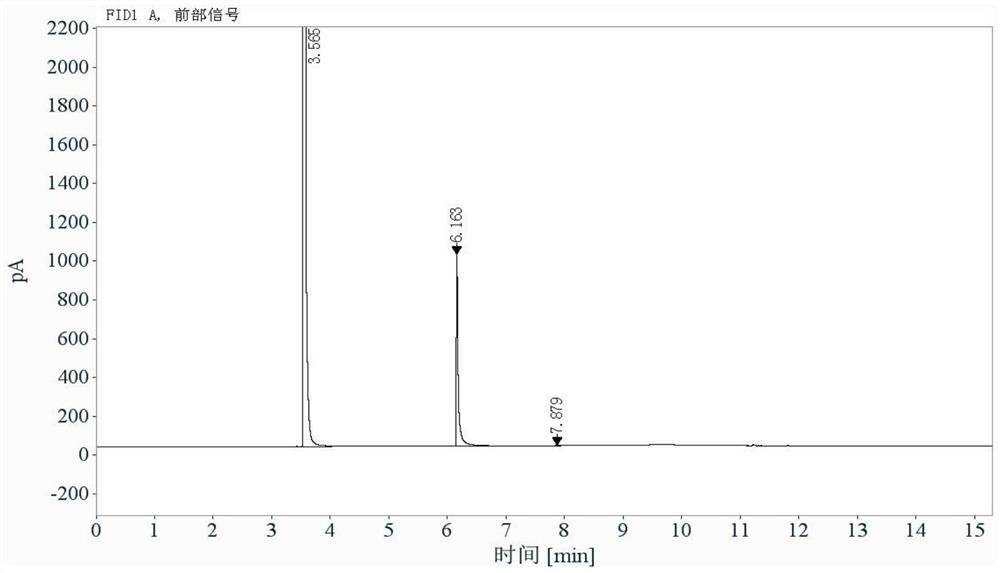
[0082] System suitability test: blank solvent, reference substance solution and test solution, inject 1 needle each, record the chromatogram, see Figu...
Embodiment 2
[0090] Preparation of the test solution: Accurately measure 5mL of the reaction solution in the preparation process of the ibrutinib bulk drug with batch number 20201103, put it in a 100mL volumetric flask, add 0.1mL of the internal standard solution, and then use methanol, N,N-di The volume ratio of methylformamide and triethylamine is a mixed solvent of 98.5:0.5:1, dissolve and dilute to the mark, shake well, and set aside.
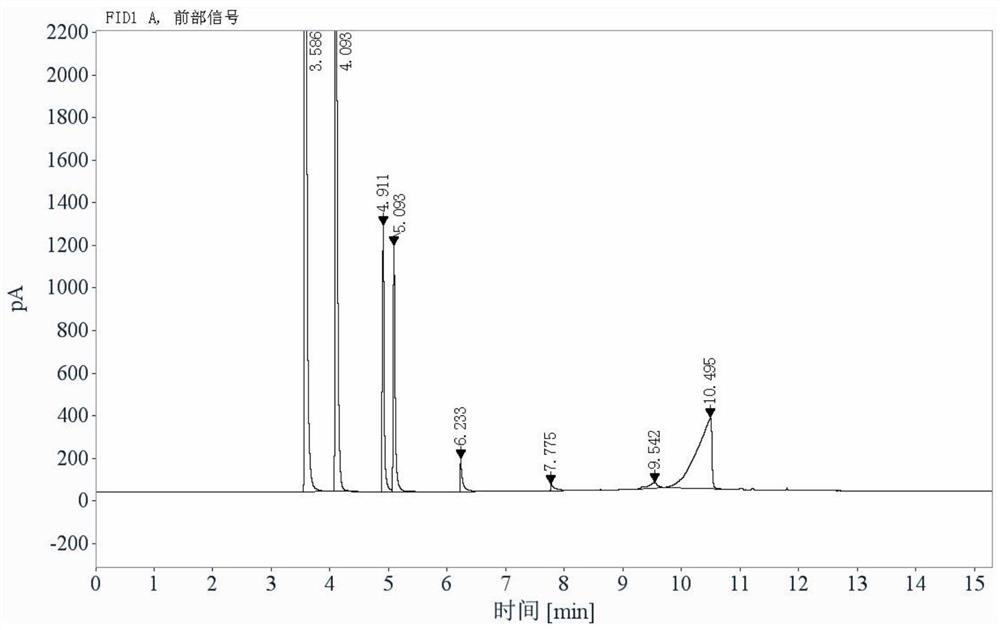
[0091] 2 parallel samples of the reference solution, 3 injections for the first sample, 2 injections for the second sample; 2 parallel samples for the test solution, 2 injections for the first sample, 2 injections for the second sample . Headspace sampling, recording chromatograms, see Figure 4 . The results showed that the detected amount of acryloyl chloride was 0.58ppm.
Embodiment 3
[0093] Preparation of the test solution: Accurately weigh 0.5g of the crude ibrutinib crude drug with batch number 20201104, put it in a 10mL volumetric flask, add 0.1mL of the internal standard solution, and then use methanol, N,N-dimethylformamide, The volume ratio of triethylamine is a mixed solvent of 98.5:0.5:1, dissolve and dilute to the mark, shake well, and set aside.
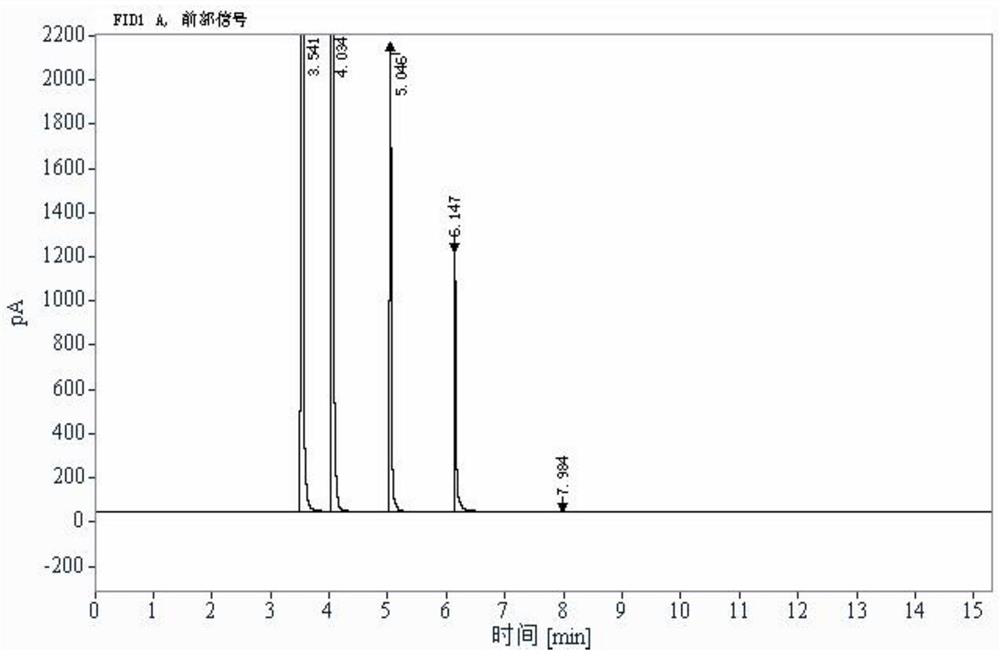
[0094] 2 parallel samples of the reference solution, 3 injections for the first sample, 2 injections for the second sample; 2 parallel samples for the test solution, 2 injections for the first sample, 2 injections for the second sample . Headspace sampling, recording chromatograms, see Figure 5 .The results showed that acryloyl chloride was not detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap