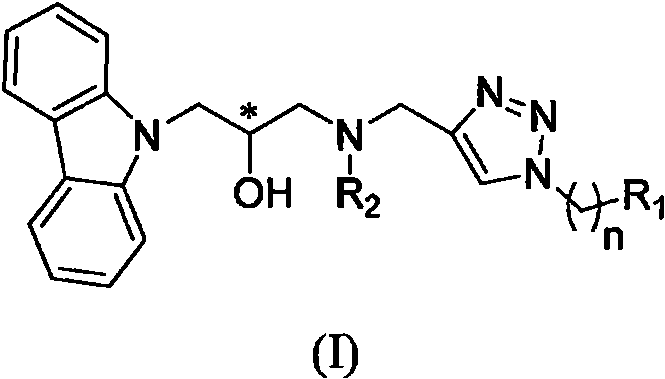
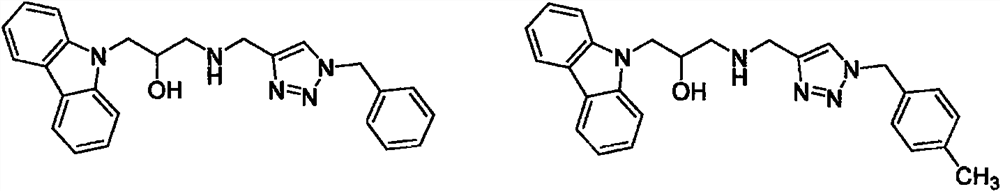
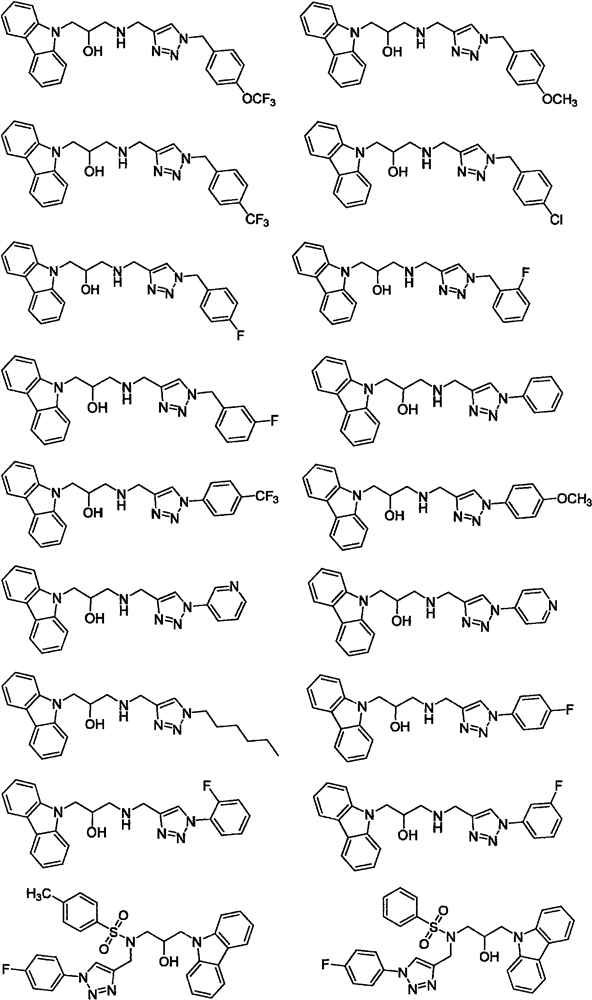
Carbazole isopropanol diamine compound containing 1, 2, 3-triazole as well as preparation method and application of carbazole isopropanol diamine compound
A carbazole isopropanol and compound technology, which is applied in the field of medicinal chemistry and can solve problems such as harmful effects on ecological environment and plant safety, and increasing drug resistance of plant pathogens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of intermediate 9-(2,3-epoxypropyl)-9H-carbazole
[0049] Carbazole (6.0mmol), KOH (0.90mmol) and 10mL DMF were added to a 100mL round-bottomed flask, stirred for 30 minutes under ice-bath conditions, then epoxybromopropane (6.0mmol) was slowly added dropwise in the system, and ice After bath reaction for 5h, end the reaction; use ethyl acetate (50mL) to extract, then use saturated NH 4 Cl aqueous solution (3×20mL), washed with anhydrous Na 2 SO 4 Drying, precipitation, and column chromatography (PE:EA=30:1, V / V) gave a white solid with a yield of 76.9%. Its NMR data are: 1 H NMR (400MHz, DMSO-d 6 , ppm) δ8.15 (d, J=7.7Hz, 2H, carbazol-H), 7.66 (d, J=8.3Hz, 2H, carbazol-H), 7.49-7.40 (m, 2H, carbazol-H), 7.22 (t, J = 7.4Hz, 2H, carbazol-H), 4.79 (dd, J = 15.7, 3.2Hz, 1H), 4.43 (dd, J = 15.7, 5.6Hz, 1H), 3.31 (dt, J = 6.8, 2.9Hz, 1H), 2.79-2.73(m, 1H), 2.58(dd, J=5.1, 2.6Hz, 1H); 13 C NMR (101MHz, DMSO-d 6 , ppm) δ140.3, 125.7, 122.1...
Embodiment 2
[0050] Embodiment 2: Preparation of intermediate 1-(9H-carbazol-9-yl)-3-(propargylamino)-2-propanol
[0051] 9-(2,3-epoxypropyl)-9H-carbazole (0.90mmol), K 2 CO 3 (0.90mmol) and 5mL of anhydrous isopropanol were added into a 25mL round bottom flask, then propargylamine (1.8mmol) was added, and reacted at 60°C, and the reaction was terminated after 6h. Desolvation, column chromatography (CH 2 Cl 2 :CH 3 OH=200:1, V / V), a white solid was obtained with a yield of 41.2%. Its NMR data are: 1 H NMR (400MHz, DMSO-d 6 , ppm) δ8.13 (d, J=7.6Hz, 2H, carbazol-H), 7.62 (d, J=8.3Hz, 2H, carbazol-H), 7.43 (t, J=7.7Hz, 2H, carbazol-H) H), 7.19 (t, J=7.4Hz, 2H, carbazol-H), 5.75 (s, 1H, NH), 5.04 (d, J=5.2Hz, 1H, OH), 4.46 (dd, J=14.7, 4.8Hz, 1H, N- CH 2 ), 4.28 (dd, J=14.7, 7.1Hz, 1H, N- CH 2 ), 4.11-3.88(m, 1H, CH), 3.36(t, J=2.2Hz, 2H, NH- CH 2 ), 3.05(t, J=2.4Hz, 1H, C·CH), 2.70-2.56(m, 2H, CH- CH 2 ); 13 C NMR (101MHz, DMSO-d 6 , ppm) δ141.1, 126.0, 122.5, 120.5, 11...
Embodiment 3
[0053] Example 3: Target compound 16 (1-(9H-carbazol-9-yl)-3-(((1-(4-fluorophenyl)-1H-1,2,3-triazole-4- base) methylene) amino) -2-propanol) preparation
[0054] 1-(9H-carbazol-9-yl)-3-(propargylamino)-2-propanol (1.08 mmol) and p-fluorophenyl azide (4.32 mmol) were dissolved in 5 mL DCM and charged to 15 mL reaction In the bottle, NaASC (0.22mmol) and CuSO 4 ·5H 2 O (0.11 mmol) was dissolved in water (0.5 mL), added to the reaction system, and stirred overnight at room temperature. TLC tracking, after the reaction is complete, add water to quench the reaction, then use CH 2 Cl 2 (50mL) extraction, take the organic phase, through anhydrous Na 2 SO 4 After drying, desolvation, and then column chromatography (CH 2 Cl 2 :CH 3 OH=200:1, V / V), a yellow solid was obtained with a yield of 48.1%. Its NMR data are: 1 H NMR (400MHz, DMSO-d 6 , ppm) δ8.63 (s, 1H, triazole-H), 8.12 (d, J=7.5Hz, 2H, carbazol-H), 7.98-7.83 (m, 2H, benzyl-H), 7.63 (d, J =8.3Hz, 2H, carbazol-H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com