Compound crystal, preparation method and application
A compound and crystal technology, applied in the field of chemistry and medicine, can solve problems such as not providing crystal structure data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
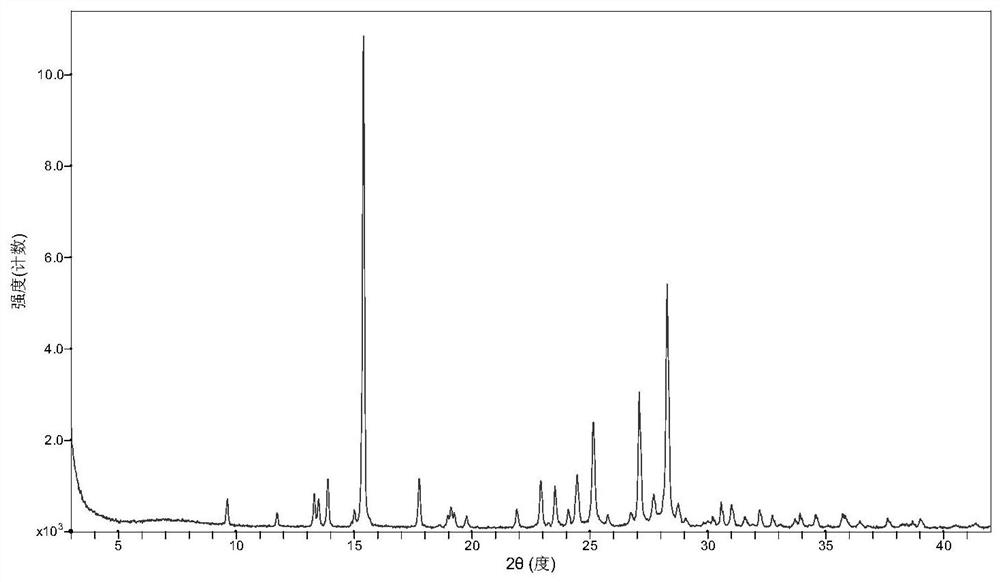
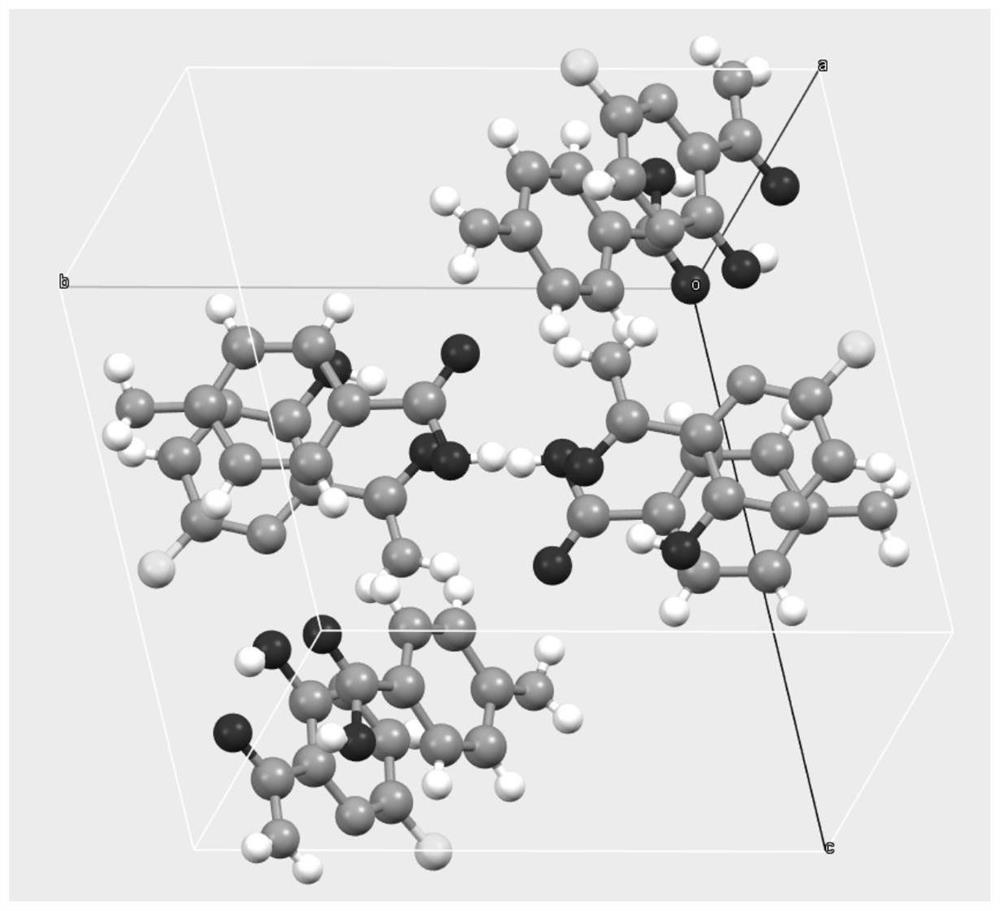
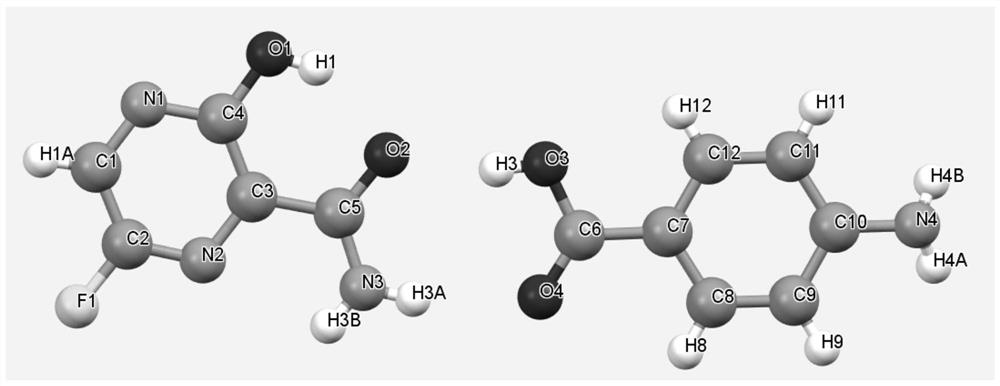
[0031] After mixing Favipiravir and p-aminobenzoic acid uniformly at a molar ratio of 1:1, take about 2 g of solid, add about 0.5 ml of ethyl acetate, and grind manually in a mortar for 30 minutes. The samples were dried in a forced air oven at 30°C. The resulting product is analyzed by XRD and is a co-crystal of Favipiravir-p-aminobenzoic acid.
Embodiment 2
[0033] After mixing Favipiravir and p-aminobenzoic acid uniformly at a molar ratio of 1:1, take about 2g of solid, add 10ml of methyl tert-butyl ether, and suspend and stir with magnetic stirring at room temperature for 72h-96h. After the sample was filtered, the obtained solid was dried in a blast drying oven at 60°C. Gained solid shows through XRD analysis, is the eutectic of favipiravir-p-aminobenzoic acid.
Embodiment 3
[0035] Take 0.6g of p-aminobenzoic acid, add 50ml of ethyl acetate, dissolve after heating. Then add 0.456g of Favipiravir and stir for 2h. After the solid was filtered, it was air-dried at 40°C. XRD analysis showed that it was a co-crystal of favipiravir-para-aminobenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com