Preparation method of exenatide microneedle
A technology of exenatide and microneedles, which is applied in the field of preparation of exenatide microneedles, can solve the problems of short half-life of exenatide and inconvenience for patients, and achieve the effect of complete needle shape and uniform drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the preparation of exenatide microneedle with PVA, 400kDa hyaluronic acid (HA) and 6000 dextran (Dextran) as material
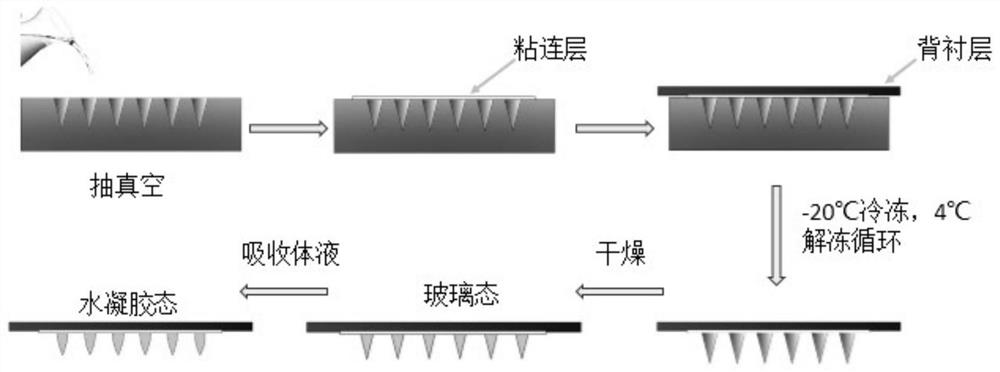
[0054] figure 1 The preparation process of exenatide phase-inversion microneedles is disclosed, that is, the polymer solution is poured on the mold with a microhole array, the polymer solution fills the micropores, and the needle tip solution is pumped into the micropores by vacuuming In the process, exenatide microneedles can be obtained after several "freeze-thaw" cycles, and the exenatide microneedles can be obtained after drying. The preparation process is simple. Specific steps are as follows:
[0055]First prepare PVA solution (10%-35% PVA aqueous solution can be selected, select 18% PVA aqueous solution for use in the present embodiment) and the mixed aqueous solution of HA and Dextran (can contain 0.5%-5% HA, 0.3%-20% in the mixed aqueous solution Dextran; the mixed aqueous solution selected in this embodiment contains 1% HA and...
Embodiment 2
[0064] Embodiment 2, release kinetics of exenatide microneedles
[0065] In order to investigate the release of exenatide, the exenatide microneedle sheet prepared in the above example was punched out with a punching device into a microneedle disc with a diameter of 12 mm (about 170 microneedles), and the microneedle sheet was made of polytetrafluoroethylene Wrapped in vinyl fluoride and tin foil, the body of the microneedle is partially exposed. Add 2ml of pH 7.4 phosphate buffer solution to the 24-well plate, put the wrapped microneedle piece into the 24-well plate with the needle tip facing down, cover it, and put it in a constant temperature shaker at 37°C and 100rpm for in vitro For release experiments, samples were taken at 0.5h, 1h, 2h, 3h, 4h, 5h, 6h, 8h, 10h, 12h, 16h, 20h, and 24h. After each sample was taken, pour out the release solution remaining in the 24-well plate, wash the 24-well plate with new PBS buffer solution, and add 2ml of the release solution to cont...
Embodiment 3
[0074] Example 3, Investigation on the Uniformity of Drug Loading of Exenatide Microneedles
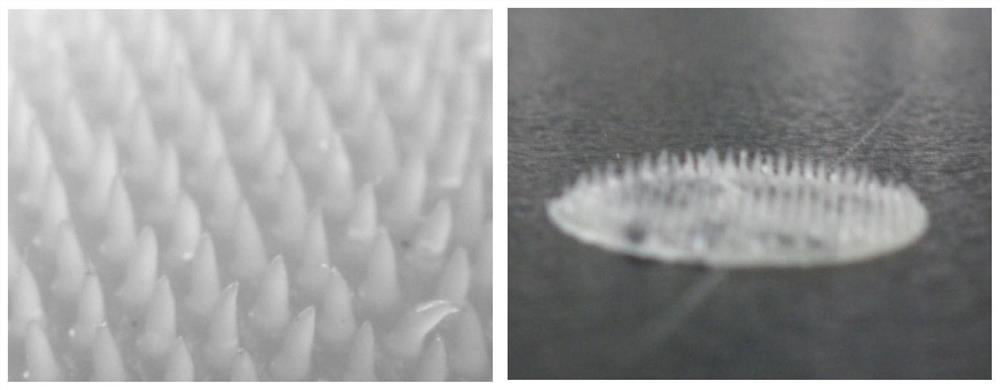
[0075] In order to verify the uniformity of dispersion of exenatide in different parts of the same mold, we investigated the uniformity of the drug loading of the microneedles. The release amount of exenatide in different parts of the microneedle tablets on the same mold was basically the same at each time point, the cumulative release curves basically overlapped, and the final in vitro release rate was about 70%. It shows that the error of in vitro release of the microneedle sheets of the same mold is small and the uniformity is good. The result is as Figure 8 ;Depend on Figure 8 It can be seen that the drug loading uniformity is good, and the drug release curves of microneedle tablets at different positions are close.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com