Preparation method of parathyroid hormone (PTH) microneedle
A micro-needle and micro-needle sticker technology, applied in the directions of micro-needles, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. The effect of swelling capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
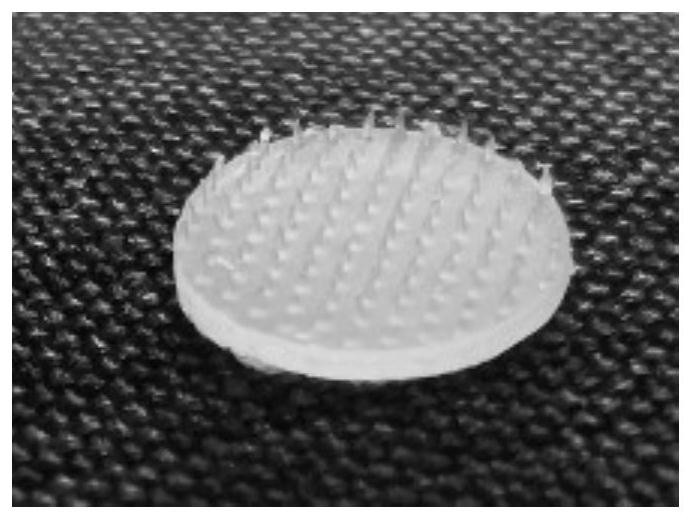
Image
Examples
Embodiment 1
[0049] Embodiment 1, take PVA, CMC-Na and Dextran as the preparation of the PTH microneedle of material
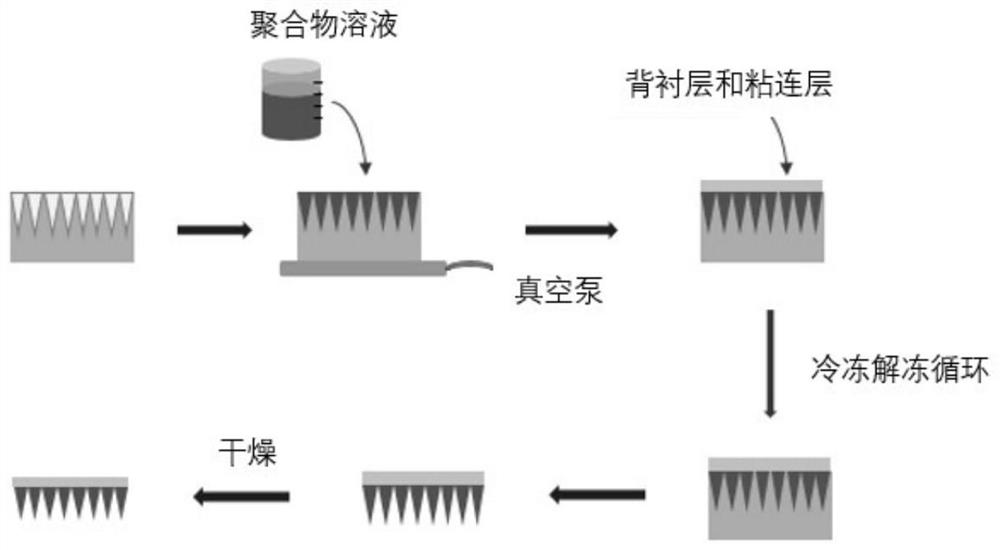
[0050] figure 1 The preparation process of PTH microneedles is disclosed, that is, the polymer solution is poured on the mold with micropore arrays, the polymer solution fills the micropores, the film is removed after freezing and thawing, and the PTH microneedles can be obtained after drying. The preparation process is simple . Specific steps are as follows:
[0051]First prepare PVA aqueous solution (10-30%, w / w, be 15% in the present embodiment) and CMC-Na (3%-10%, w / w sodium carboxymethyl cellulose, be 5% in the present embodiment ) and Dextran (dextran 3%-10%, w / w, 7% in the present embodiment) mixed aqueous solution, according to a certain ratio (PTH, PVA aqueous solution, dextran and sodium carboxymethylcellulose mixed The mass ratio of the three in the aqueous solution is 1 / 6 / 3) Fully mix the PTH and the above two solutions. Then the prepared medicinal solution...
Embodiment 2
[0058] The release kinetics of embodiment 2, PTH microneedle
[0059] In order to investigate the release of PTH, the microneedles prepared above were placed in a Franz diffusion cell device, the release solution was PBS with pH = 7.4, stirred at 100 rpm at 37°C, the top of the microneedles was covered with a plastic film, and samples were taken at regular intervals. ELISA kit was used to measure PTH concentration. After calculation, the cumulative release curve of PTH is obtained, the result is as follows Figure 5 ;Depend on Figure 5 It can be seen that the drug release process is stable.
Embodiment 3
[0060] Example 3, Investigation on the Uniformity of Drug Loading of PTH Microneedles
[0061] The PTH microneedles are loaded with PTH in the polymer solution. In order to verify the uniformity of PTH dispersion in the solution, we investigated the uniformity of the drug loading of the microneedles. Put the three pieces of microneedles into the PBS buffer solution with pH=7.4, place them in 37→water-bath shaker for 3h, shake well after the end, take the leaching solution, and use the PTH-ELISA kit to measure the leaching solution of the microneedles respectively. The result obtained is as Figure 6 ;Depend on Figure 6 It can be seen that the drug loading uniformity is good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com