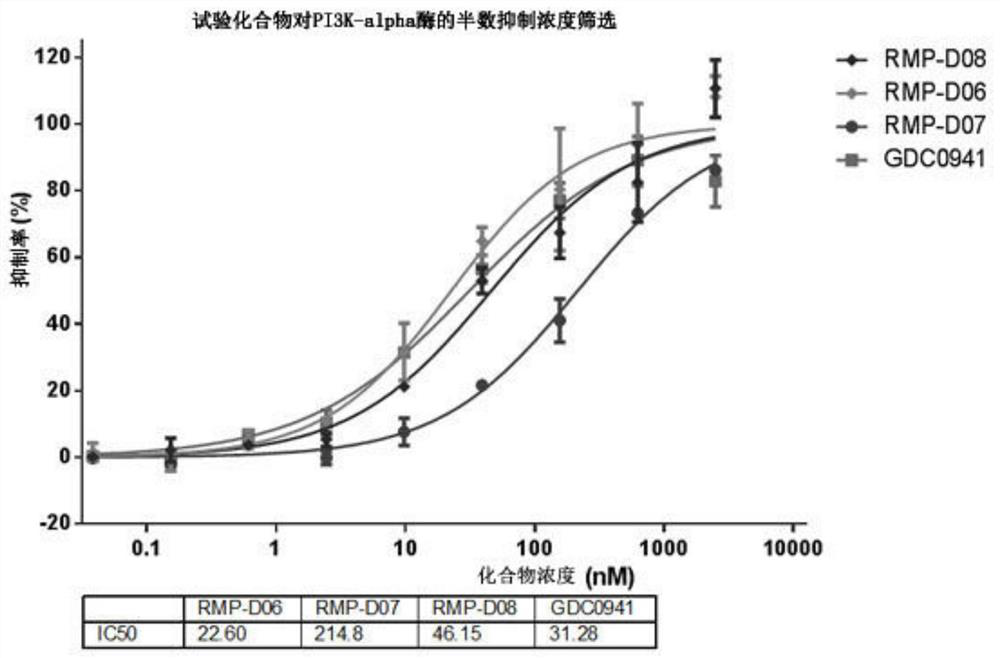
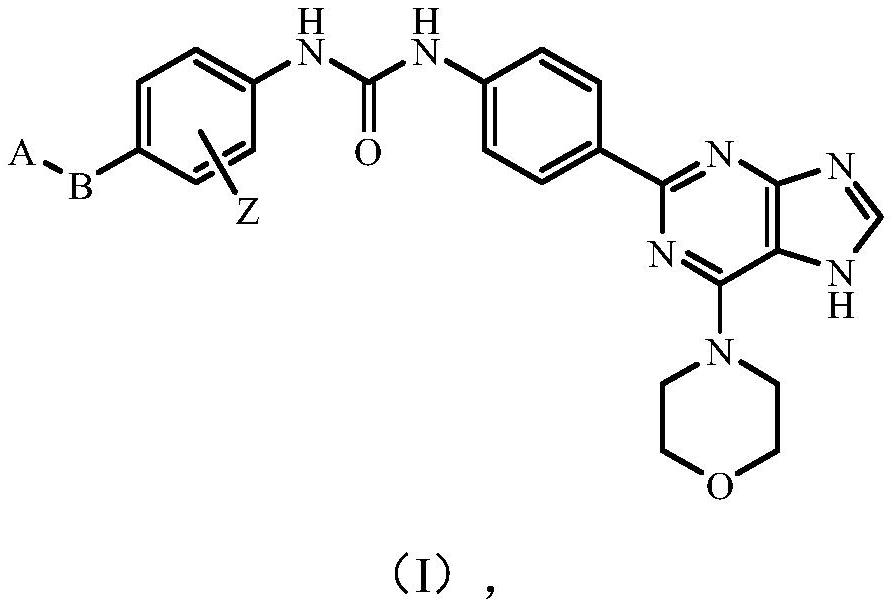
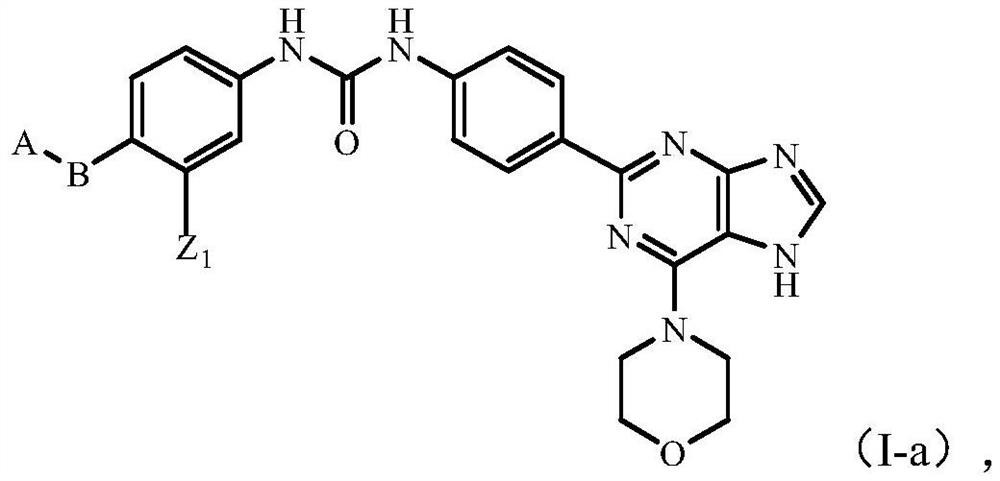
Novel purine derivative, intermediate thereof and application thereof in preparation of anti-cancer drugs
A technology of derivatives and intermediates, applied in the field of novel purine derivatives and their intermediates, to achieve the effect of low preparation cost, simple structure, and excellent PI3K inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 compound I-1
[0058] Take the following route to synthesize compound I-1:
[0059]
[0060] 1.1. Synthesis of compound 2
[0061] Compound 2: Chinese name is 2-(4-isocyanatophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxolane; English name is 2-(4-isocyanatophenyl )-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
[0062]
[0063] Add 4-aminophenylboronic acid pinacol ester compound 1 (10g, 45.6mmol) and triethylamine (13.8g, 136.8mmol) respectively into 300mL of dichloromethane, cool to zero degree, slowly add triphosgene in batches at zero degree (8.1 g, 27.4 mmol), and then stirred at zero for 50 minutes to obtain a solution of compound 2, which was directly used in the next step.
[0064] 1.2. Synthesis of compound 3
[0065] Compound 3: The Chinese name is 1-(4-(hydroxymethyl)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxin-2- base) phenyl) urea; the English name is:
[0066] 1-(4-(hydroxymethyl)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2...
Embodiment 2
[0085] The preparation of embodiment 2 compound 1-2
[0086] Take the following route to synthesize compound I-2:
[0087]
[0088] 2.1. Synthesis of Compound 9
[0089] Compound 9: The Chinese name is (4-nitro-2-(trifluoromethyl)phenyl)methanol; the English name is:
[0090] (4-nitro-2-(trifluoromethyl)phenyl)methanol;
[0091]
[0092] Compound 8 (25.0g, 106.4mmol) was dissolved in tetrahydrofuran (100mL), sodium borohydride (11.5g, 319.1mmol) was slowly added in an ice bath, and boron trifluoride diethyl ether (20mL) was slowly added dropwise, followed by stirring at room temperature overnight. TLC showed that after the reaction was over, 100 mL of brine was added, extracted with ethyl acetate (300 mL x 3), and the organic phase was dried and concentrated over sodium sulfate to obtain compound 9 (14.5 g, yield = 61%). Measured: ESI- MS m / z=222[M+1] +
[0093] 2.2. Synthesis of compound 10
[0094] Compound 10: The Chinese name is tert-butyldimethyl(4-nitro-...
Embodiment 3
[0115] The preparation of embodiment 3 compound 1-3
[0116] Take the following route to synthesize compound I-3:
[0117]
[0118] 3.1. Synthesis of compound 15
[0119] Compound 15: The Chinese name is 4-(2-(benzyloxy)ethyl)morpholine; the English name is:
[0120] 4-(2-(benzyloxy)ethyl)morpholine;
[0121]
[0122] Compound 14 (32.8g, 0.25mol) was dissolved in DMF (200mL), NaH (10g, 0.25mol) was added in portions under cooling in an ice-water bath, stirred at room temperature for 1.5 hours, benzyl bromide (39.3g, 0.23 mol). The reaction solution was stirred at room temperature for 16 hours, concentrated under reduced pressure, added ethyl acetate (100 mL), washed with water and saturated brine. The organic phase was concentrated under reduced pressure and purified by silica gel column (DCM:MeOH=10:1) to obtain compound 15 (40 g, yield 78%) as a colorless oil. Measured: ESI-MS m / z=222[M+1] +
[0123] 3.2. Synthesis of Compound 16
[0124] Compound 16: Chine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com