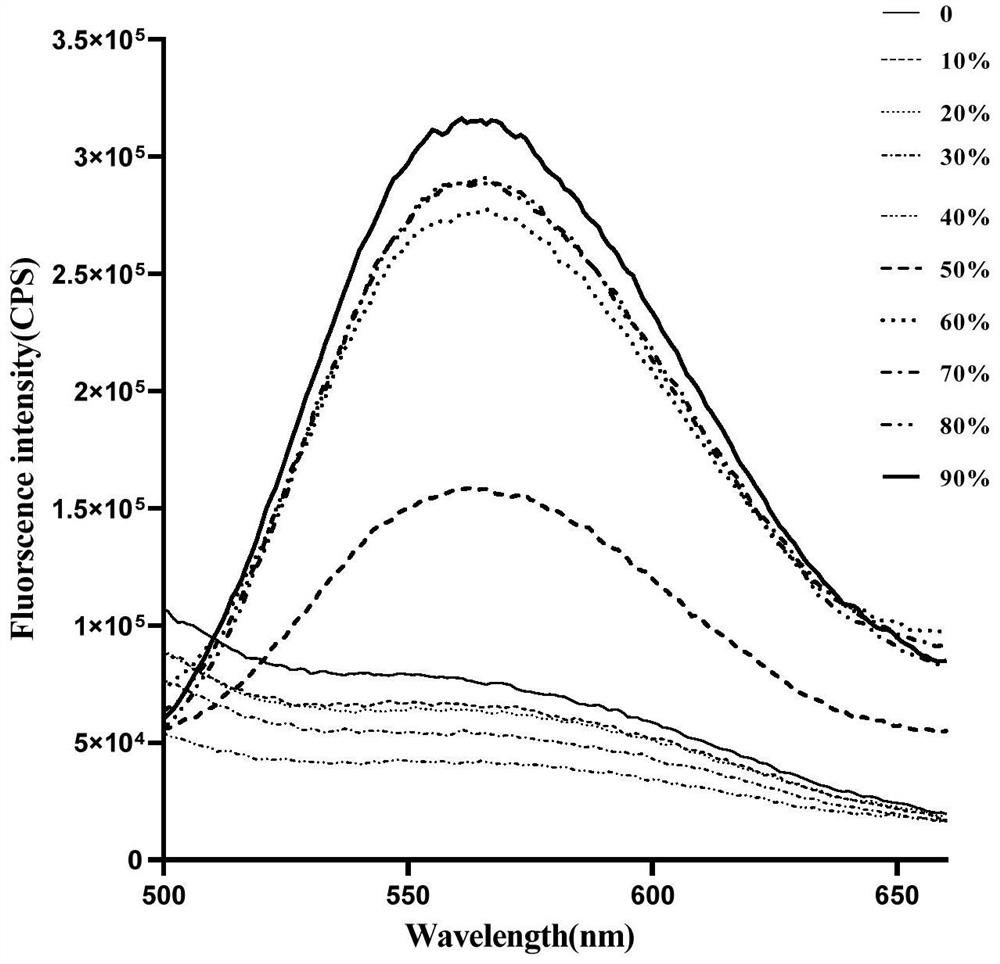
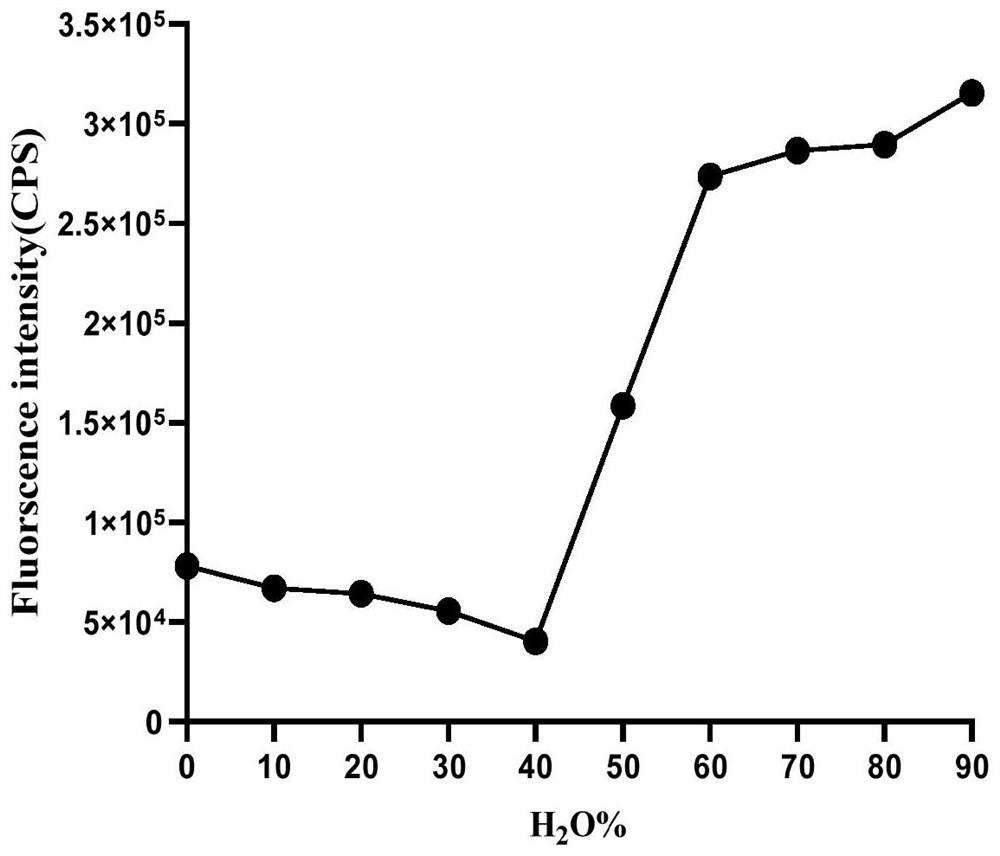
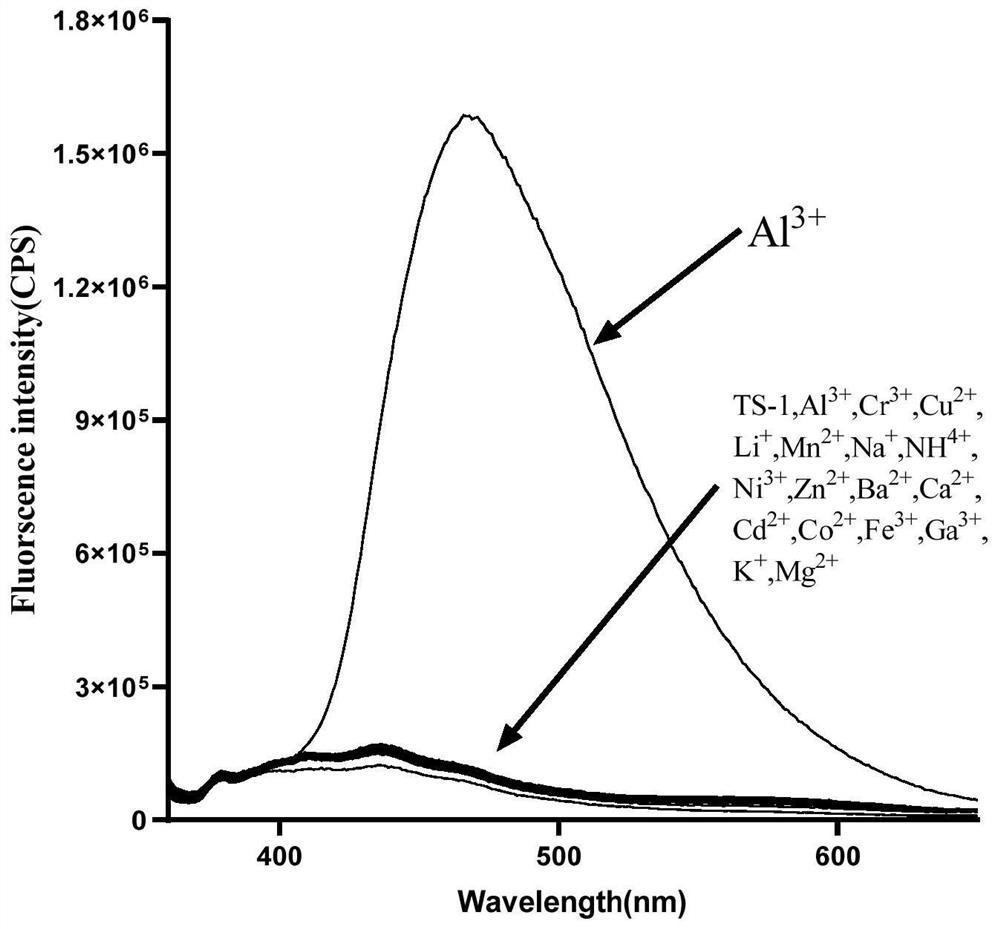
A Class of Cationic Fluorescent Probes Based on Tetraphenylethylene Structure
A tetraphenylethylene, fluorescent probe technology, applied in fluorescence/phosphorescence, luminescent materials, material analysis by optical means, etc., can solve problems such as limiting practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The synthetic route of compound TS-1 is as follows:
[0056]
[0057] Synthesis of compound 1
[0058] Take 30mL redistilled tetrahydrofuran in a reaction flask, under the protection of argon, add zinc powder (5.2g, 80mmol) into the redistilled THF, stir for 15min under ice bath, slowly add TiCl dropwise with a syringe 4 (4.5mL, 40mmol), heated to reflux for 2h. Dissolve benzophenone (1.82g, 10mmol) and 4,4'-dihydroxybenzophenone (2.57g, 12mmol) in 20mL redistilled tetrahydrofuran, slowly add to the reaction flask, continue to heat and reflux overnight, McMurry couple occurs couplet. After the reaction, with saturated NaHCO 3 Quench the reaction with the solution, adjust the pH to 7, extract three times with 30mL dichloromethane, combine the organic phases, wash with water, anhydrous Na 2 SO 4 dry. Silica gel column chromatography (200-300 mesh column chromatography silica gel, petroleum ether:ethyl acetate / V:V=3:1) gave compound 1 (pale white solid, 1.93g, yie...
Embodiment 2
[0065] The preparation method was the same as that of compound TS-1 in Example 1, except that benzohydrazide was replaced by salicylhydrazide to obtain compound TS-2 (yellow solid, yield 85.2%).
[0066] 1 H NMR (300MHz, DMSO-d 6 )δ11.94(s,2H),11.75(s,2H),11.13(s,2H),8.51(s,2H),7.88(dd,J=7.9,1.7Hz,2H),7.46(ddd,J =8.5,7.2,1.6Hz,2H),7.18(ddd,J=17.1,9.2,3.8Hz,8H),7.07–6.92(m,10H),6.74(d,J=8.5Hz,2H).
[0067]
Embodiment 3
[0069] The synthetic route of compound TS-3 is as follows:
[0070]
[0071] Synthesis of compound 3
[0072] Take 60mL redistilled tetrahydrofuran in a reaction flask, under the protection of argon, add 9.6g zinc powder (9.6g, 0.147mol) into the redistilled THF, stir for 15min under ice bath, slowly add TiCl dropwise with a syringe 4 (8mL, 0.072mol), stirred at room temperature for 0.5h, heated to 70°C, and refluxed for 2.5h. Cool to room temperature again, add 3 mL of pyridine under ice-bath conditions, stir for 10 min, dissolve benzophenone (2.62 g, 14.4 mmol) and 4-hydroxybenzophenone (2.85 g, 14.4 mmol) in 20 mL redistilled THF, Slowly added to the reaction flask, continue heating to reflux overnight. After the reaction, with saturated NaHCO 3 Quench the reaction with the solution, adjust the pH to 7, extract three times with 30mL dichloromethane, combine the organic phases, wash with water, anhydrous Na 2 SO 4 dry. Silica gel column chromatography (200-300 colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com