Attenuated vaccine strain of canine adenovirus type 2 and its application
A technology of canine adenovirus and attenuated vaccines, which is applied in the direction of viruses, vaccines, antiviral agents, etc., can solve the problems of poor pertinence and high cost of vaccines, and achieve the effects of long duration of immunity, strong acid resistance, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
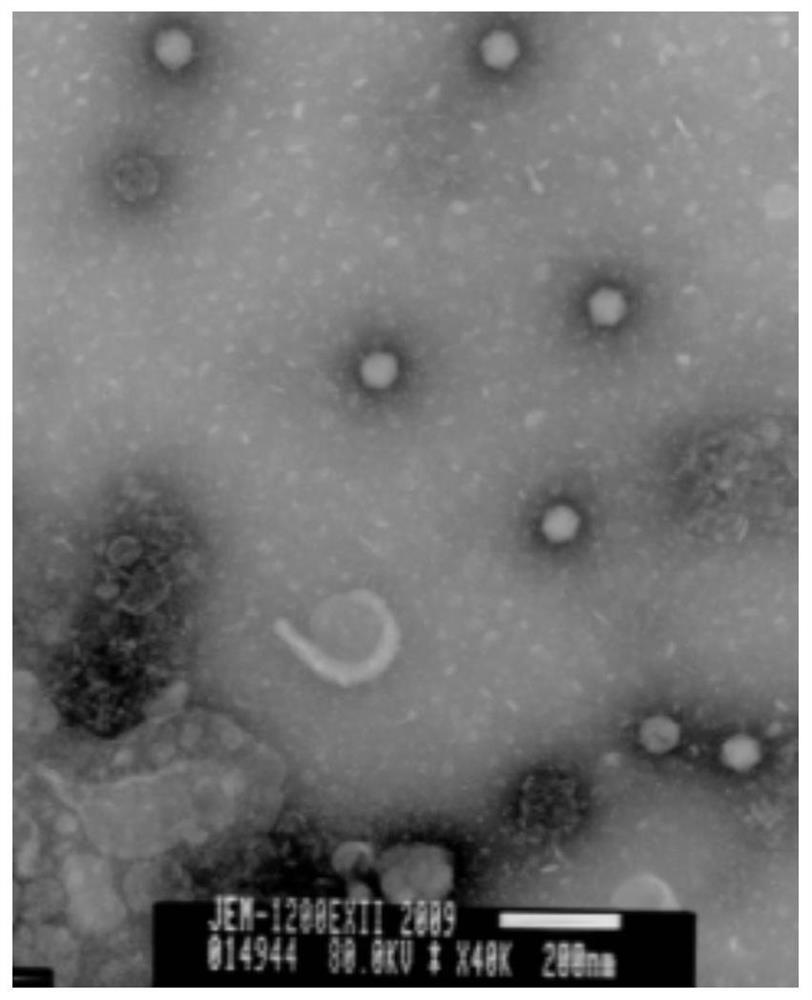
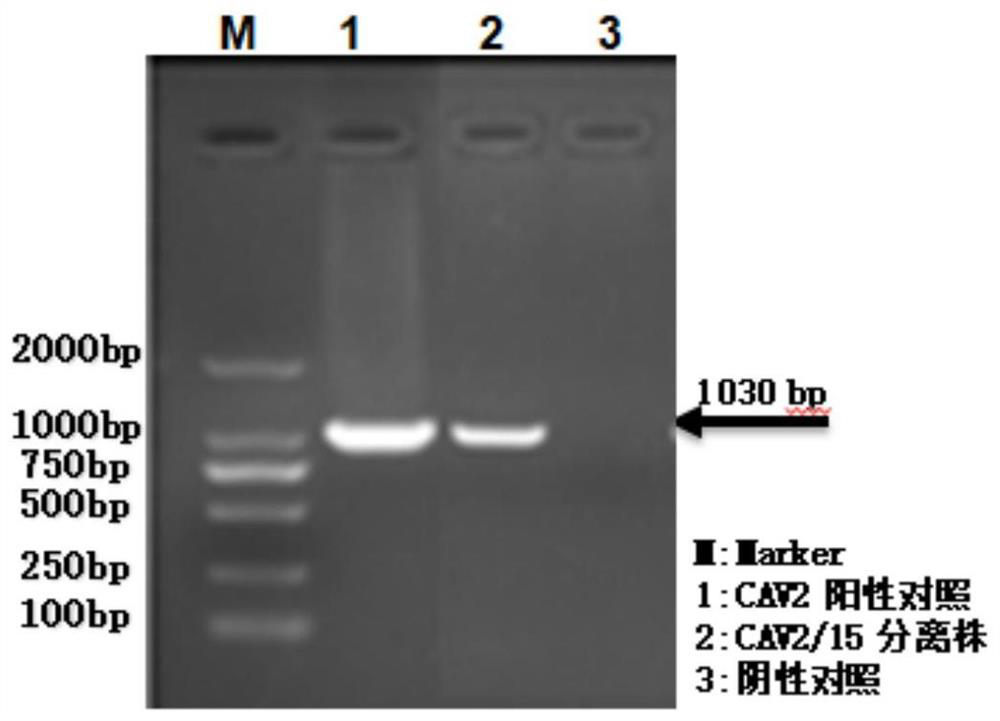
[0044] Example 1 Isolation and identification of canine adenovirus natural attenuated CAV2 / 15 strain.
[0045] Virus isolation: Most of the weaned puppies from a dog farm in Shenyang City, Liaoning Province had clinical symptoms such as mild cough and serous nasal discharge, and were diagnosed as suspected canine adenovirus disease. There were no obvious pathological changes in anatomy. Tonsils, lungs and hilar lymph nodes were collected from dogs to isolate the virus.
[0046] Cells: Canine kidney passaged cells MDCK (passage 87) cell line was purchased from China Veterinary Drug Administration, and kept and supplied by Beijing Shengtaier Technology Co., Ltd.
[0047]Experimental animals: 42 to 49-day-old healthy beagle dogs that were not immunized with canine adenovirus vaccine and were negative for antigen and antibody were purchased from Shenyang Kangping Laboratory Animal Research Institute.
[0048] Serum: canine CAV2 standard positive serum (SN≥1:512), negative serum, ...
Embodiment 2
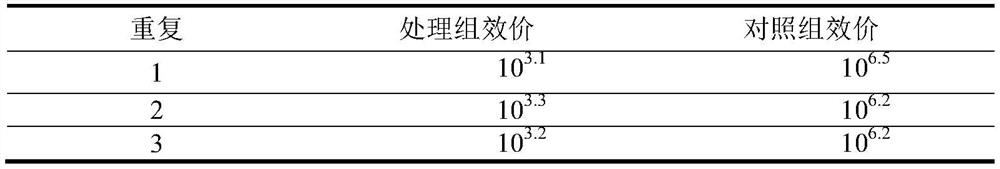
[0087] Example 2 Canine Passaging Reversion Test
[0088] Test Materials and Methods
[0089] Cells: Canine kidney passaged cells (MDCK), purchased from China Veterinary Drug Administration, preserved and provided by Beijing Shengtaier Technology Co., Ltd.
[0090] Virus species: Canine adenovirus type 2 virus CAV2 / 15 strain, F50 generation; preserved and provided by Beijing Shengtaier Technology Co., Ltd.
[0091] Experimental animals: The experimental dogs were 42-49-day-old healthy dogs with negative CAV2 antigen and antibody that had not been immunized with canine adenovirus type 2 vaccine.
[0092] 1. Cell passage stability test: use MDCK to passage cells, adopt the method of simultaneous inoculation, culture at 37 °C, and harvest if more than 80% cytopathic (CPE) occurs within 4 to 5 days of culture, and then continue to culture and passage with MDCK to 50%. The changes of cells at each passage (CAV2 / 15F50) were observed, and the virus titer of each passage was determi...
Embodiment 3
[0105] Example 3 Preparation of canine adenovirus type 2 live vaccine (CAV2 / 15 strain)
[0106] Preparation of attenuated antigen of canine adenovirus type 2 (CAV2 / 15 strain): CAV2 / 15 strain virus strains were inoculated into MDCK cell monolayer at a ratio of 2%, cultured at 37°C for 4 to 5 days, and freeze-thawed when the cytopathic effect reached more than 80% Harvest virus fluid.
[0107] Determination of virus titer in culture: take the cultured virus liquid as 10 4 , 10 5 , 10 6 , 10 7 , 10 8 , 10 9 Diluted and inoculated into a 96-well cell culture plate with a good monolayer of MDCK cells, each dilution inoculated into 4 wells, 0.1 mL / well, and set virus and normal cell controls at the same time, set at 37 ℃, 5% CO 2 Incubate for 4 to 5 days, observe and record CPE every day, and calculate TCID according to the Reed-Muench method 50 , virus culture virulence ≥ 10 9.0 TCID 50 / mL.
[0108] The preparation of canine adenovirus type 2 live vaccine (CAV2 / 15 strain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com