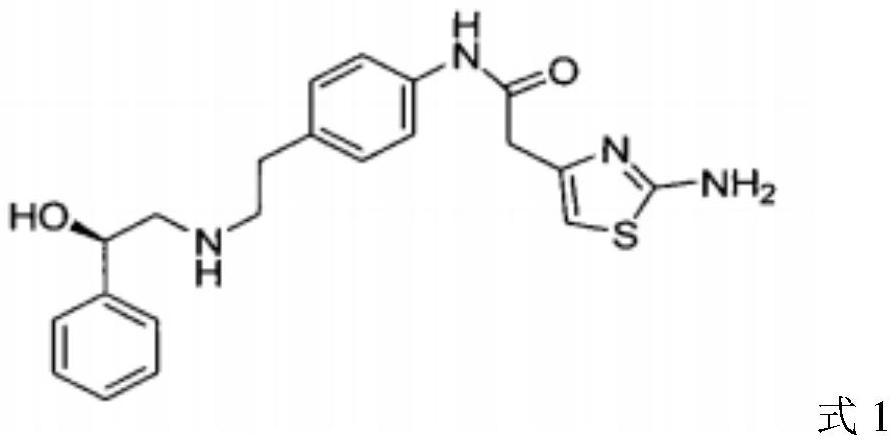
Preparation method of mirabegron intermediate
A technology of intermediates and compounds, which is applied in the field of preparation of Mirabegron intermediates, can solve problems such as unfavorable industrial production, high price, and not being environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
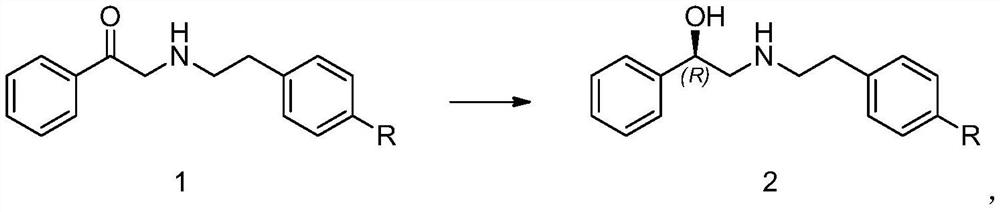
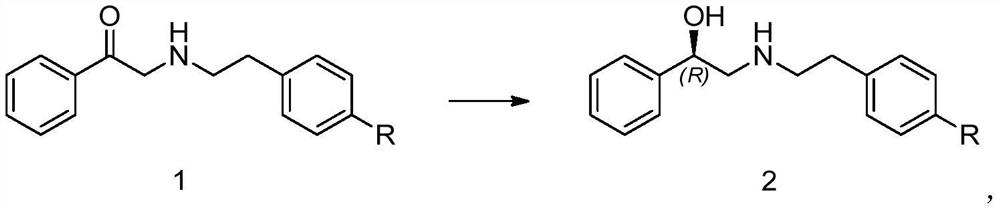
Embodiment 1
[0040] The preparation of embodiment 1 compound 2-1
[0041]
[0042] At room temperature, add 20mg of compound 1-1 and 16.85mg of D-glucose into 3ml of 0.2M phosphate buffered saline with pH=6.7, add 10mg of ketoreductase ET-027, 5mg of coenzyme II, and 5mg of glucose dehydrogenase, at 20°C Magnetic stirring at -30°C, adding saturated sodium carbonate aqueous solution dropwise to control pH = 6-8, liquid phase detection after 18 hours showed that the remaining raw materials were 0.24%, the purity of compound 2-1 was 95.80%, and the isomer impurity (S)-2 of the compound -(4-nitrophenethylamino)-1-phenylethanol was 0.19%, and the optical purity of compound 2-1 was 99.81%; 5ml of ethyl acetate was added to the system, extracted and separated, and concentrated to obtain compound 2-1 : White solid 16mg, yield 89.6%, detection:
[0043] 1H NMR (400MHz, DMSO) δ9.31 (d, J = 170.0Hz, 2H), 8.20 (d, J = 8.3Hz, 2H), 7.57 (d, J = 8.6Hz, 2H), 7.46-7.35 ( m, 4H), 7.34-7.26(m, 1H), 6.25...
Embodiment 2
[0044] The preparation of embodiment 2 compound 2-2
[0045]
[0046] At room temperature, add 20mg compound 1-2, 13.21mg D-glucose to 400ul pH=6.7 0.2M phosphate buffered saline, add 5mg ketoreductase ET-027, 5mg coenzyme Ⅱ, 5mg glucose dehydrogenase, at 20℃ Stir magnetically at -30°C, add sodium carbonate aqueous solution dropwise to control pH = 6-8, after 18 hours, there is no raw material remaining in the liquid phase test, the purity of the target product is 92.45%, the isomer impurity of compound 2-2 (S)-2- The content of (4-aminophenethylamino)-1-phenylethanol is 0.15%, and the optical purity of compound 2-2 is 99.85%. Add 2ml of ethyl acetate to the system, extract and separate, and concentrate to obtain compound 2-2: white Solid 14mg, yield 89.4%, detection:
[0047] 1H-NMR (400MHz, DMSO): δ: 2.87-2.90 (2H, m); 2.97-3.16 (4H, m); 5.06-5.09 (3H, m); 6.22 (1H); 6.55-6.57 (2H, d , J=7.7Hz); 6.88-6.91 (2H, d, J=7.7Hz); 7.29-7.42 (5H, m); 9.22 (1H, br).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com