Human Anti-il-33 monoclonal-antibody-containing pharmaceutical composition
A technology for monoclonal antibodies and compositions, applied in the field of pharmaceutical compositions containing antibodies, can solve problems such as insufficient antibodies, and achieve the effects of stable long-term storage and excellent efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0261] Example 1: Cloudiness suppression effect by adding sugar
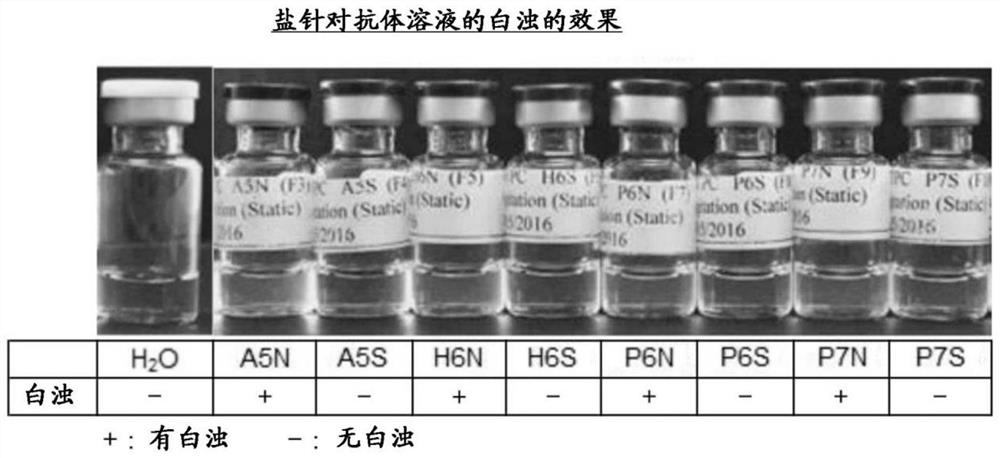
[0262] In order to eliminate the cloudiness of the human anti-IL-33 monoclonal antibody (A10-1C04, A23-1A05, A25-2C02, A25-3H04, or A26-1F02), instead of adding 5% sorbitol, as Comparative Example 2, The following formulations obtained with sodium chloride contained in formulations A4N, A5N, H6N, P6N, P7N, and P8N were evaluated. The antibody concentration was set at 150 mg / ml.
[0263] 10 mM sodium acetate / pH4 / 5% (w / v) sorbitol / 0.02% (w / v) polysorbate 80 (hereinafter referred to as "A4S")
[0264] 10 mM sodium acetate / pH5 / 5% (w / v) sorbitol / 0.02% (w / v) polysorbate 80 (hereinafter referred to as "A5S")
[0265] 10 mM histidine / pH6 / 5% (w / v) sorbitol / 0.02% (w / v) polysorbate 80 (hereinafter referred to as "H6S")
[0266] 10 mM sodium phosphate / pH6 / 5% (w / v) sorbitol / 0.02% (w / v) polysorbate 80 (hereinafter referred to as "P6S")
[0267] 10 mM sodium phosphate / pH7 / 5% (w / v) sorbitol / 0.02% (w / v) polysorbate 80 (her...
Embodiment 2
[0274] Example 2: Salt concentration and cloudiness (1)
[0275] Human anti-IL-33 monoclonal antibodies (A10-1C04, A23-1A05, A25-2C02, A25-3H04 or A26-1F02) dissolved in 10 mM histidine / pH6.0 or pH5.5 buffer were determined Also, the interaction parameter (Kd value) as an index of cohesion when 0, 50, and 100 mM NaCl is added is shown. The measurement temperature was set at 25° C., and the antibody concentrations were prepared so as to be 0.5, 1, 2.5, 5, 10, and 20 mg / mL. As a result, when the NaCl addition amount was 50 mM or more, the Kd value was a negative number (agglomeration increased), so it was considered that the NaCl addition amount should be less than 50 mM (for example, about A10-1C04, shown in Table 4). It should be noted that the Kd value can be calculated as follows: the diffusion coefficient (Dm) is obtained by the dynamic light scattering method, the slope is obtained from the coordinate diagram of the antibody concentration (horizontal axis) and the diffu...
Embodiment 3
[0280] Example 3: Salt concentration and cloudiness (2)
[0281] 0, 5, 10, and 30 mM NaCl were added to 10 mM histidine / pH6.0 / 3.6% (w / v) sorbitol, and the Kd value, which was an index of cohesion, was measured. Antibodies used A10-1C04 at 2.5, 5, 10, and 14 mg / mL. As a result, the Kd value was negative when the amount of NaCl added was above 30mM (the Kd values of 0, 5, 10, and 30mM NaCl were 17.3, 7.3, 1.2, and -4.0mL / g, respectively). Less than 30mM. The Kd value was calculated by the method described in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com