Antihypertensive pharmaceutical composition as well as preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
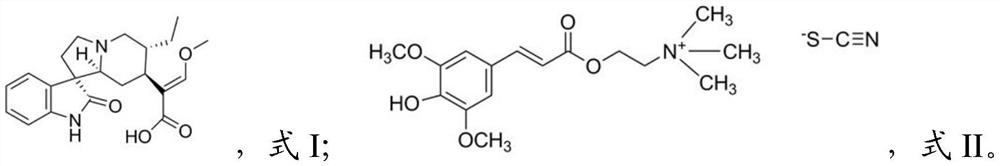
[0020] The present invention also provides a method for preparing the pharmaceutical composition described in the above technical solution, comprising the following steps: mixing isorhynchophylline and sinapine thiocyanate to obtain the pharmaceutical composition.
[0021] In the present invention, the preparation method of the isorhynchophylline preferably includes: cold-soaking Uncaria in 70% ethanol aqueous solution by volume, filtering the filtrate, concentrating under reduced pressure until it has no alcohol smell, and adjusting the pH value to 1.0~3.0, more preferably 2.0, to obtain the first extract, the first extract is mixed with chloroform according to (1~2):(1~2), more preferably 1:1 volume ratio and extract , take the water phase to adjust the pH value to 10.0~12.0, more preferably 11.0, to obtain the second extract, and the second extract is according to (1~2):(1~2), more preferably 1:1 volume Mix with chloroform, extract, take the organic phase, recover under red...
Embodiment 1
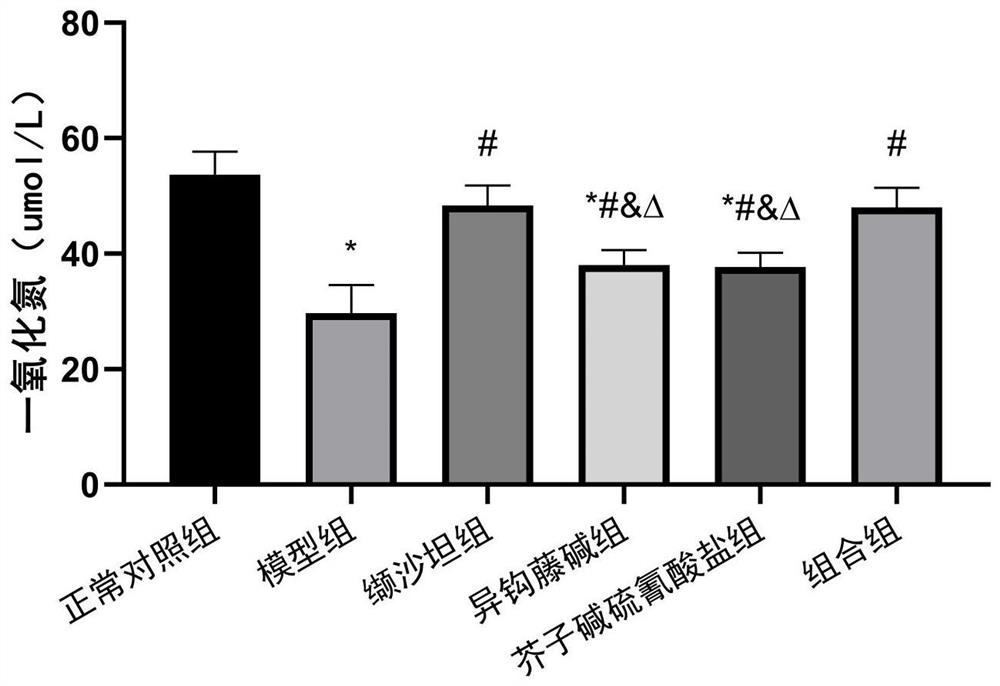
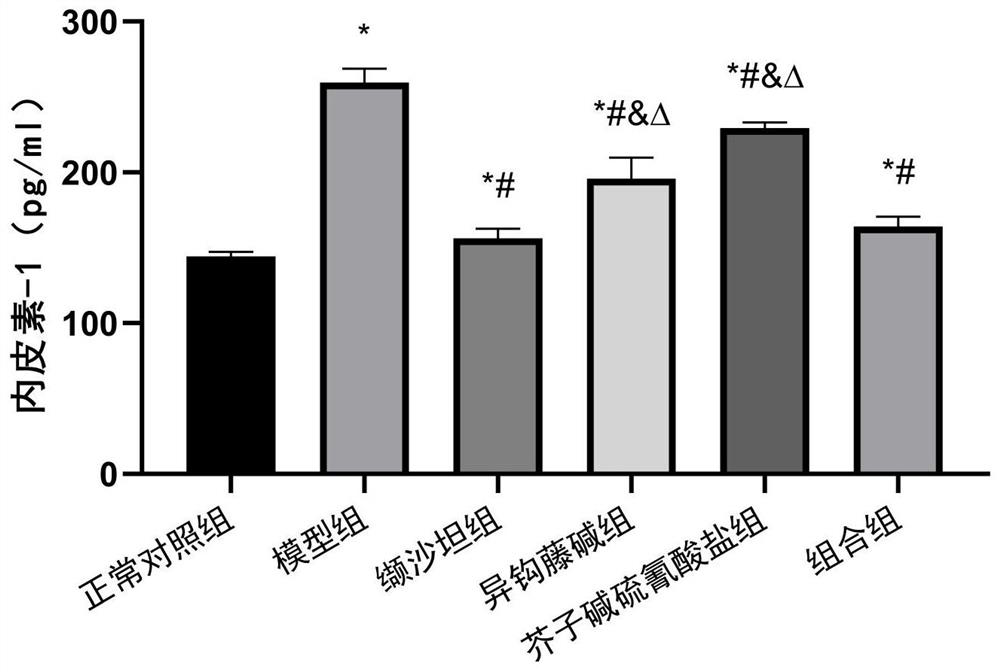
[0030] Synergistic protective effects of isorhynchophylline and sinapine thiocyanate against angiotensin II-induced HUVECs cell injury
[0031] Experimental Materials
[0032] Experimental cells: human umbilical vein endothelial cells (Human Umbilical Endothelial Cells, HUVECs), purchased from ScienCell (USA), the 4th to 7th passages were used for experiments.
[0033] Experimental drugs: Isorhynchophylline (IRN), sinapinethiocyanate (Sinapinethiocyanate, ST), and valsartan (Valsartan, Val) were purchased from Chengdu Chromar Biotechnology Co., Ltd. Angiotensin II (Angiotensin II, Ang II) was purchased from Beijing Suolaibao Technology Co., Ltd.
[0034]Experimental reagents: nitric oxide (NO) (product number: A013-2-1) was purchased from Nanjing Jiancheng Bioengineering Institute, endothelin 1 (ET-1) enzyme-linked immunoassay (ELISA) kit (product number: JYM0710Hu) was purchased from The Cell Proliferation and Toxicity Detection (CCK-8) Kit (Product No.: MAO218-1) was purch...
Embodiment 2
[0054] Antihypertensive Pharmacodynamic Experiment of Isorhynchophylline and Sinapine Thiocyanate Composition on Spontaneously Hypertensive Rats (SHR)
[0055] Experimental Materials
[0056] Experimental animals: the model group was SPF grade 8-week-old male spontaneously hypertensive rats (SHR), with a body weight of 180-200 g, and the normal group was SPF-grade 8-week-old male Wistar Kyoto rats (WKY), with a body weight of 180-200 g. All were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (SCXK (Beijing): 2016-0005), and were kept in an animal laboratory at a room temperature of 20-25°C and a humidity of 50-65%.
[0057] Experimental drugs: isorhynchophylline and sinapine thiocyanate, both purchased from Chengdu Cromar Biotechnology Co., Ltd., production batch number 20201011, specification 2g / bottle; valsartan, production batch number x1041 H2020723, Beijing Novartis Pharmaceutical Co., Ltd. product.
[0058] Experimental equipment: BP-98A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com