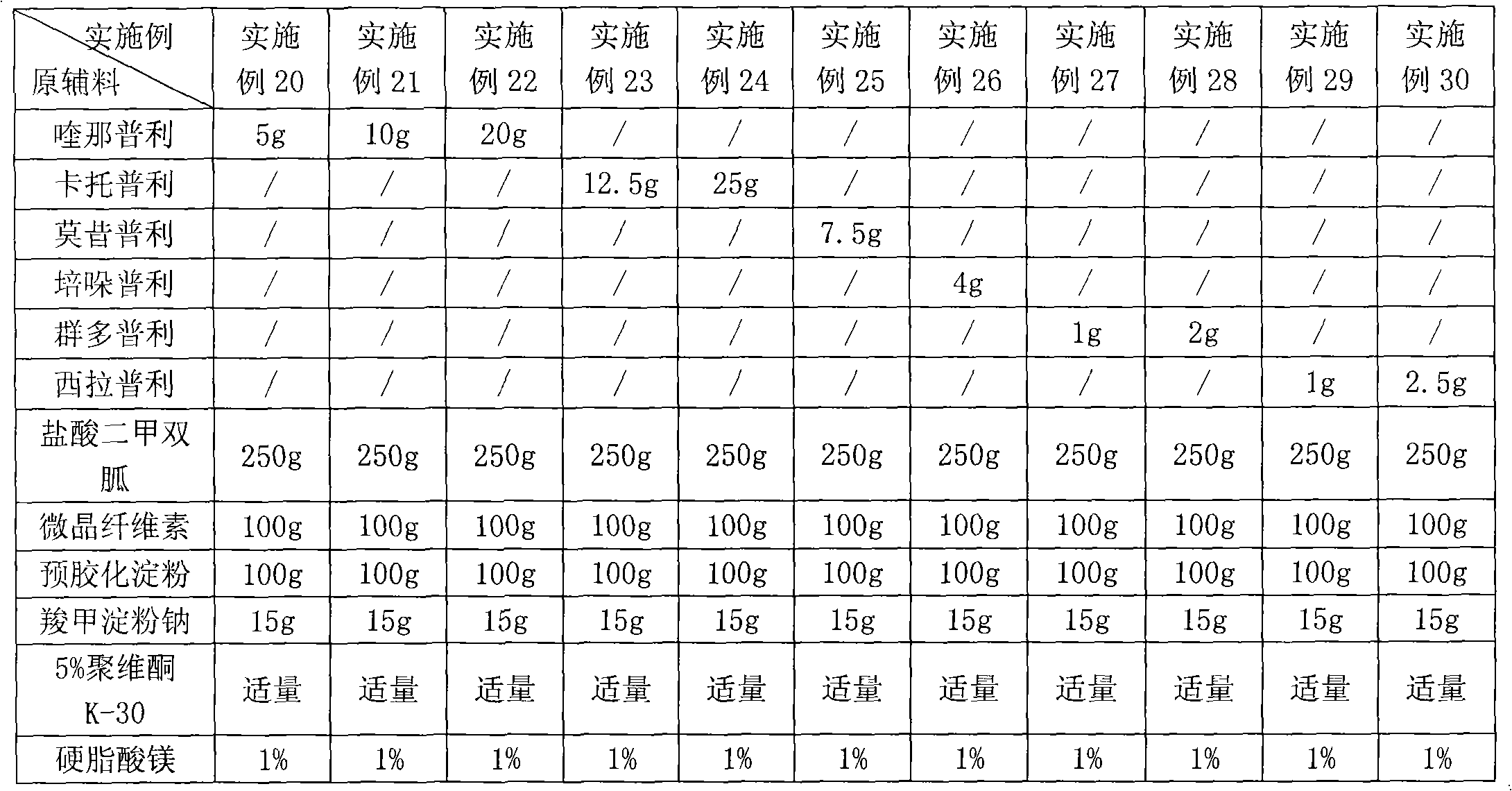
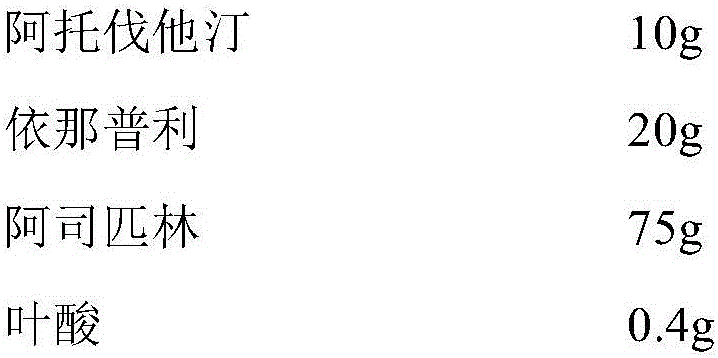Patents
Literature
81 results about "Antihypertensive medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
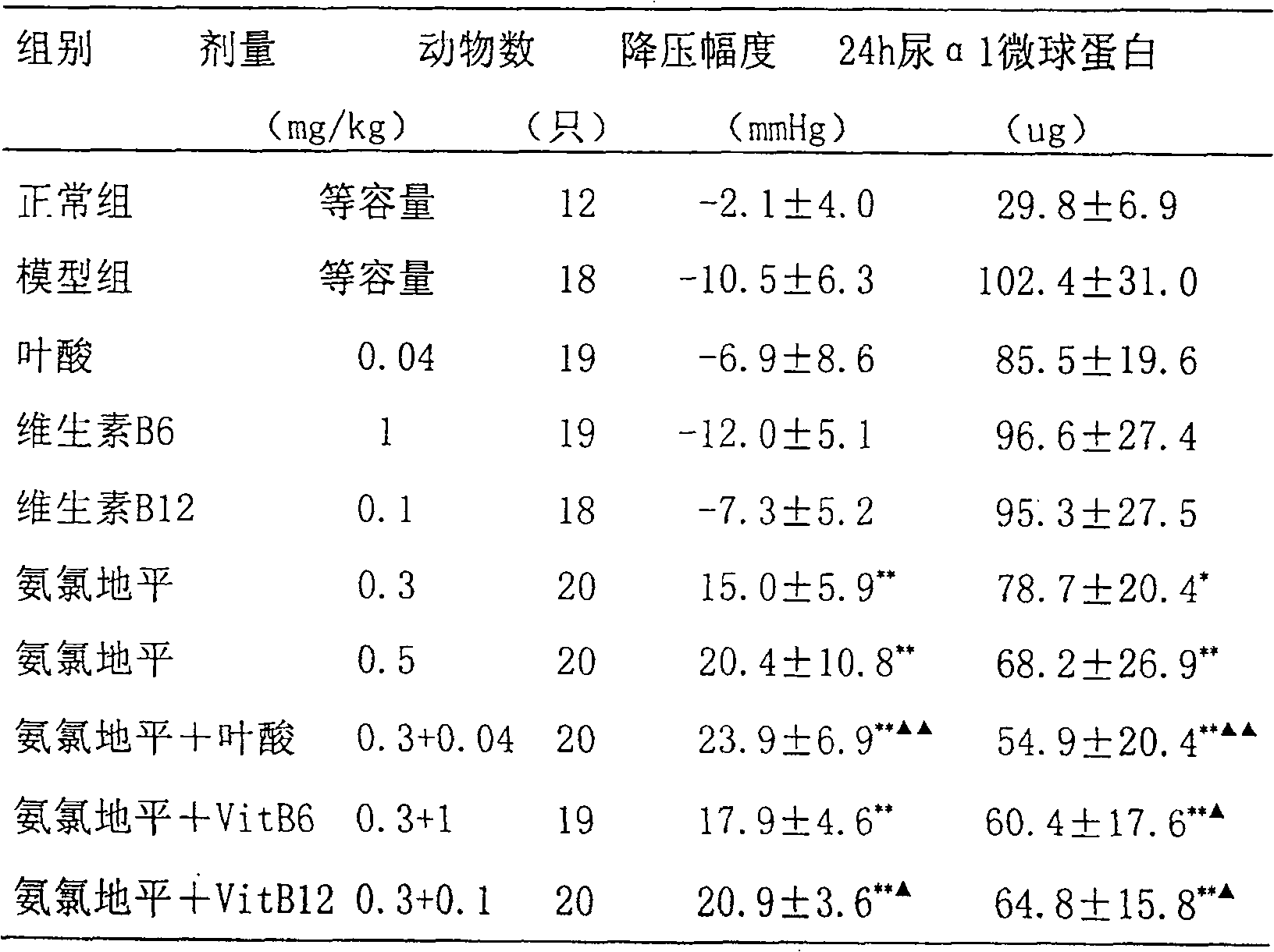
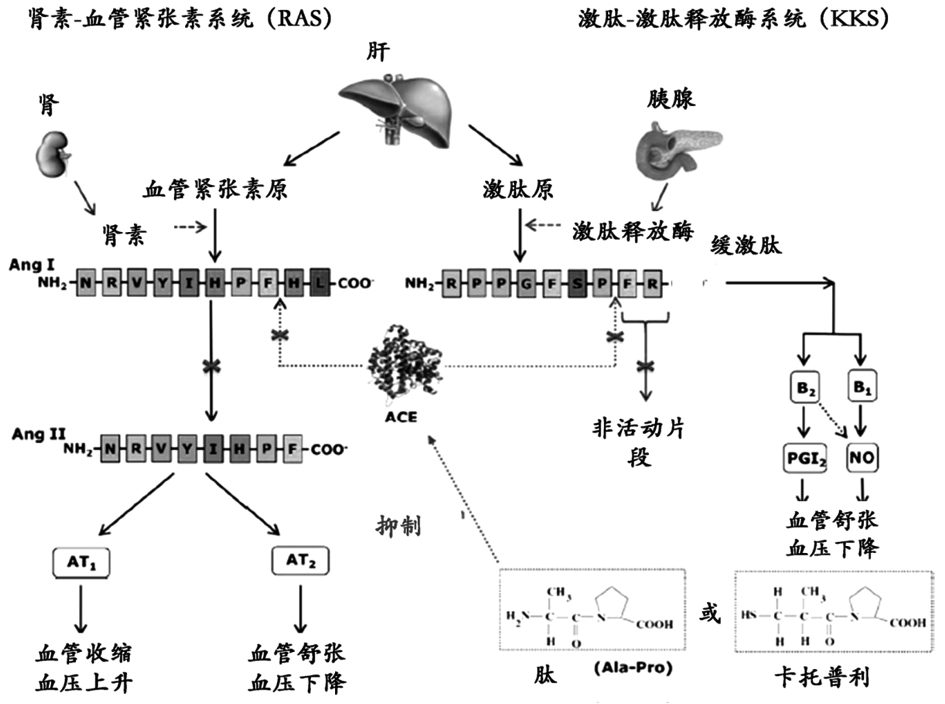
Antihypertensives are medications that lower blood pressure, reducing strain on, and damage to, the heart and blood vessels. Drugs called ACE inhibitors and angiotensin-2 receptor antagonists are used to treat high blood pressure (hypertension), as are beta blockers, calcium channel blockers, diuretics, and other medications.
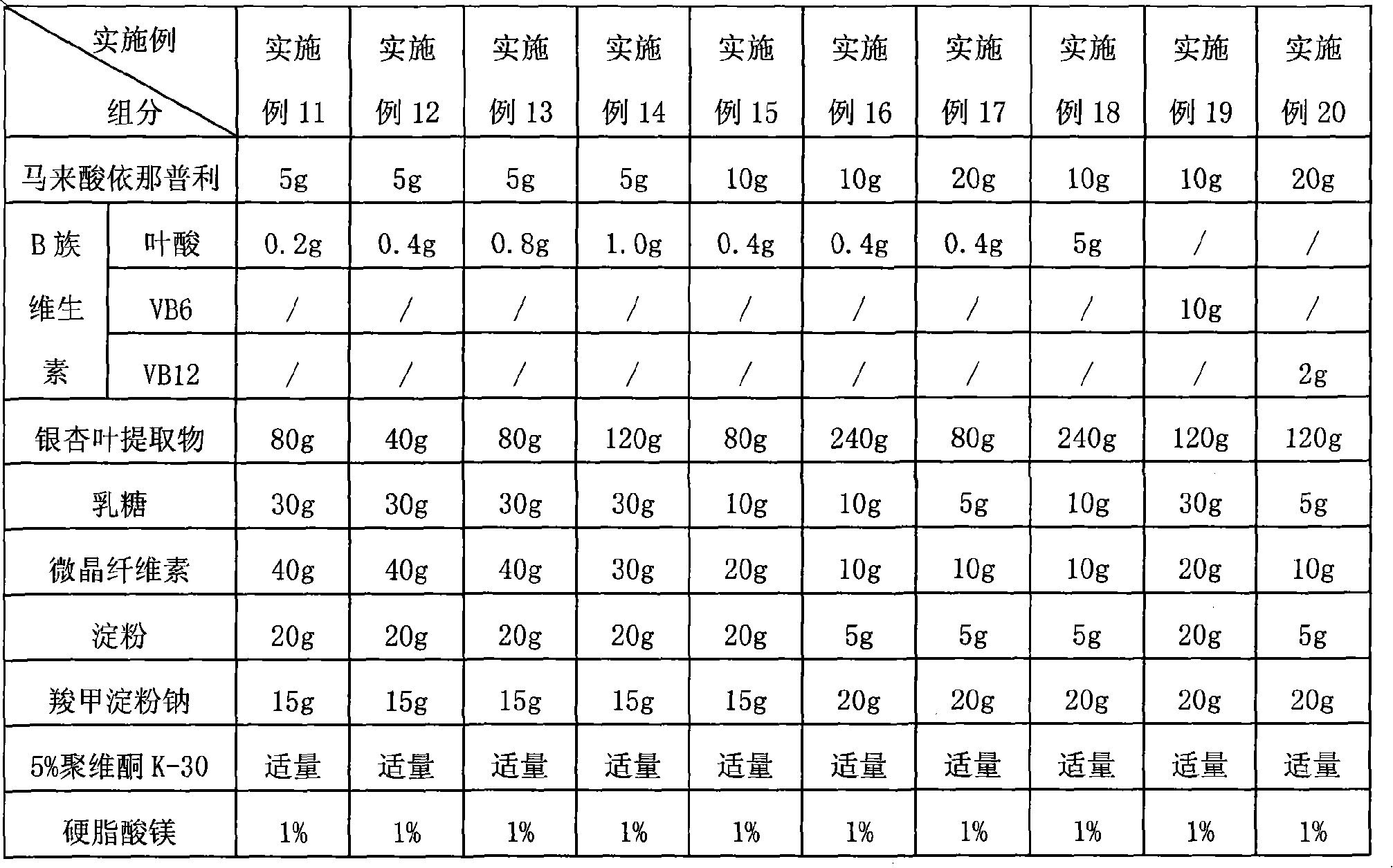
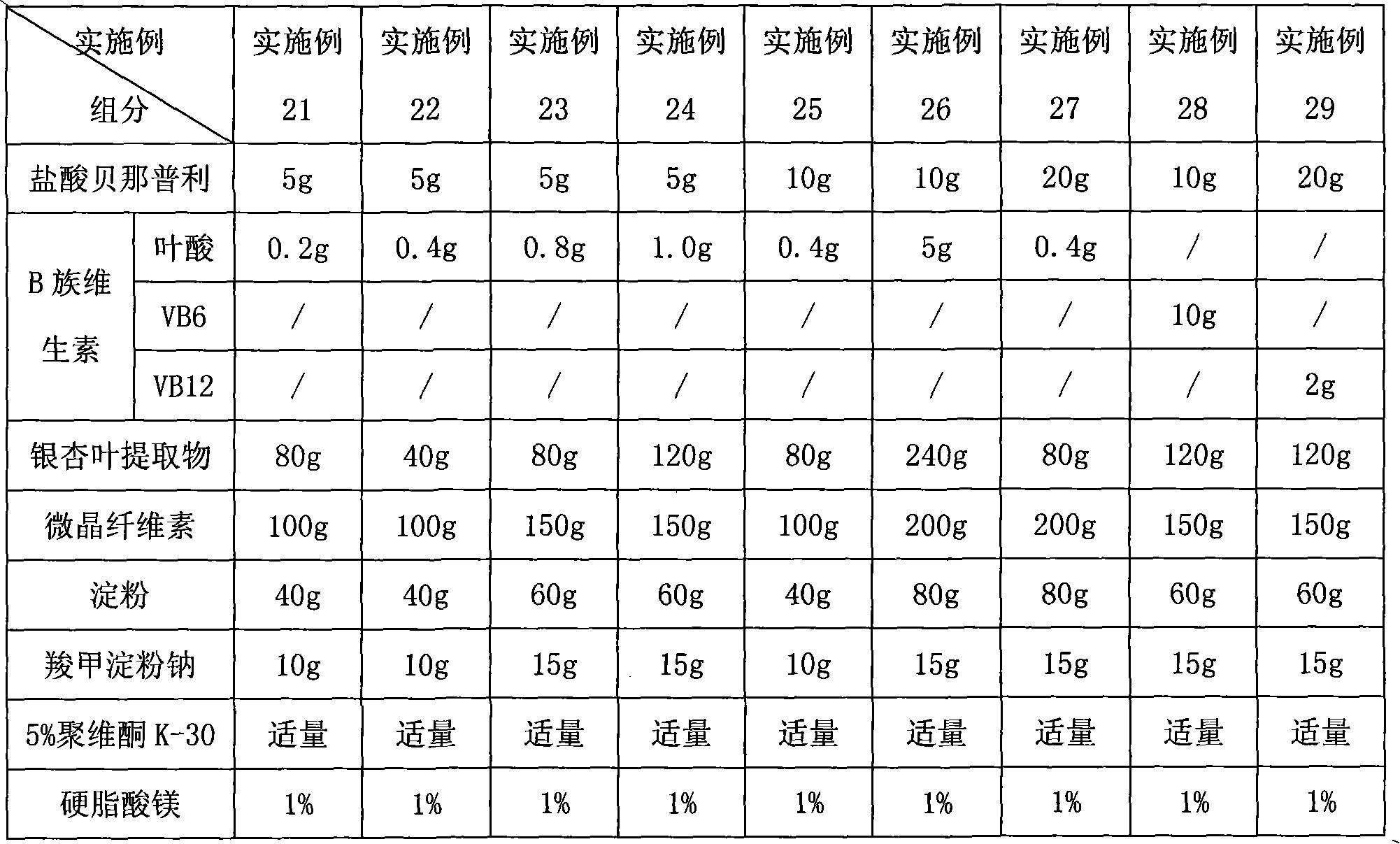
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Pharmaceutical Combinations Comprising Specified Age Breaker and Further Drugs, I.A. Antihypertensive Drugs, Antidiabetic Drugs Etc.
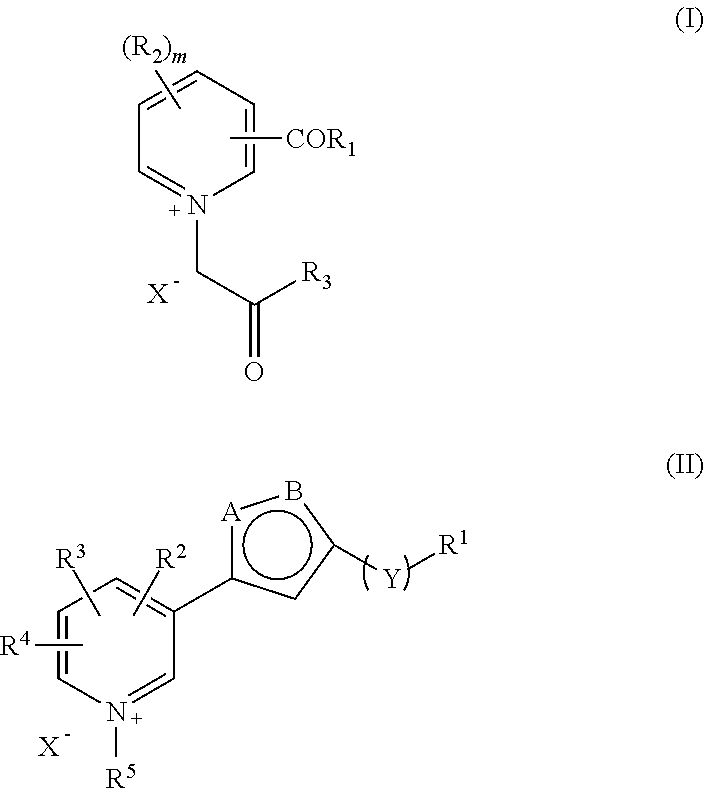
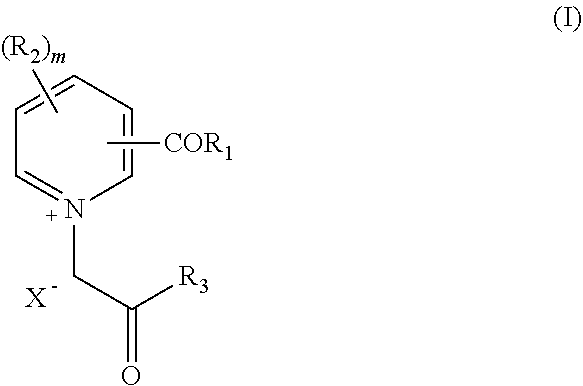
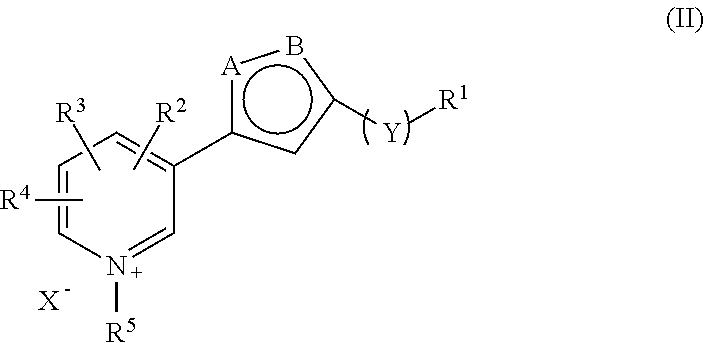
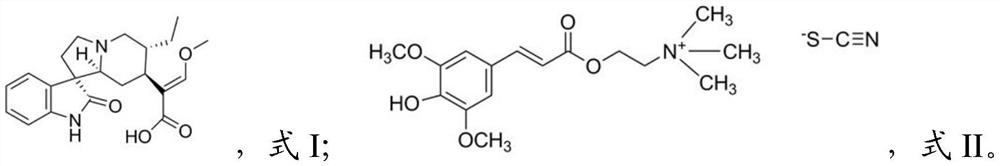
The present invention relates to a combination, such as a combined preparation or pharmaceutical composition comprising: (a) compound of formula (I), and / or (II) or a pharmaceutically acceptable salt thereof; (b) at least one therapeutic agent selected from the group consisting of: an antihypertensive agent; an antidiabetics agent; a hypolipidemic agent; an antiplatelet agent; an antiobesity agent; an antithrombotic agent; an agent for diabetic vascular complications; and an agent for treatment of heart failure; or a pharmaceutically acceptable salts thereof, optionally in presence of a pharmaceutically acceptable carrier for separate, simultaneous or sequential use. The present invention also relates to a use of such combination for the treatment of mammal including human being. R1, R2, R3, R1, R2, R3, R4, R5, X, Y, A and B and m are as defined in the specification.
Owner:TORRENT PHARMA LTD
Pharmaceutical composition containing angiotensin converting enzyme inhibitors, B vitamins and ginkgo biloba extracts
InactiveCN101590084AAppropriate treatmentPrevent or delay risk factorsOrganic active ingredientsPharmaceutical delivery mechanismDiseaseHypotensive drug
The invention relates to a pharmaceutical composition containing one of angiotensin converting enzyme inhibitors (ACEI) and active metabolites or medical salt thereof in medicine dosage, one or more of B vitamins in medicine dosage, ginkgo biloba extracts (GbE) in medicine dosage and a medical carrier, and relates to application of the pharmaceutical composition in preparing medicines for treating, preventing or retarding stroke. The medicines prepared by the pharmaceutical composition provided by the invention are superior to the simple antihypertensive drugs in the aspect of treating, preventing or retarding stroke and facilitate the patients to take. The invention belongs to the pharmaceutical field.
Owner:SHENZHEN AUSA PHARM CO LTD
Chinese medicine preparation for antihypertension and invigorating pulse
InactiveCN1435242AReduce aggregationEffective treatmentUnknown materialsCardiovascular disorderCurative effectRhizome
A Chinese medicine for treating hypertension and preventing cerebral infarction is prepared from 17 Chinese-medicinal materials including Chinese angelica root, Chuan-xiong rhizome, red sage root, peach kernel, etc through proportioning. Its advantages are high curative effect and no by-effect.
Owner:张以芳
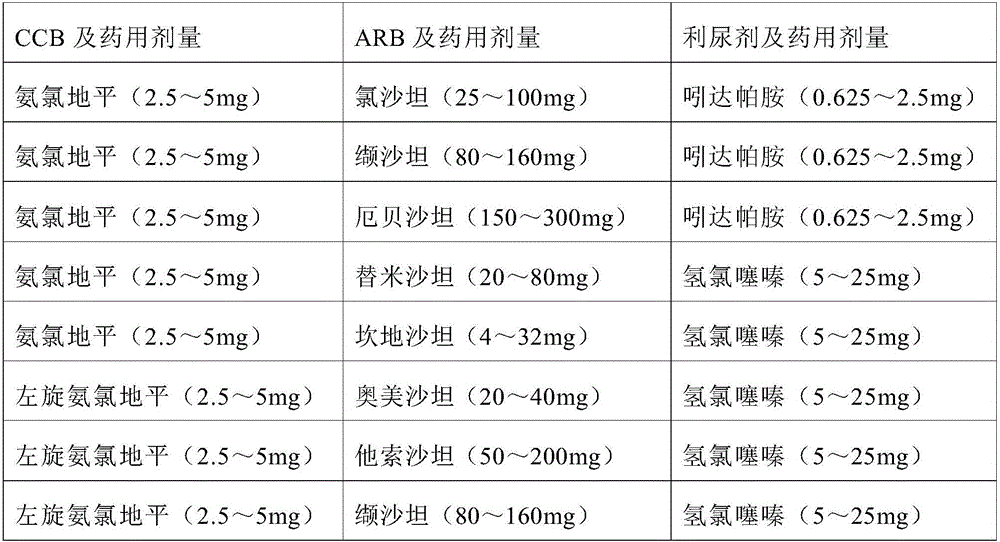
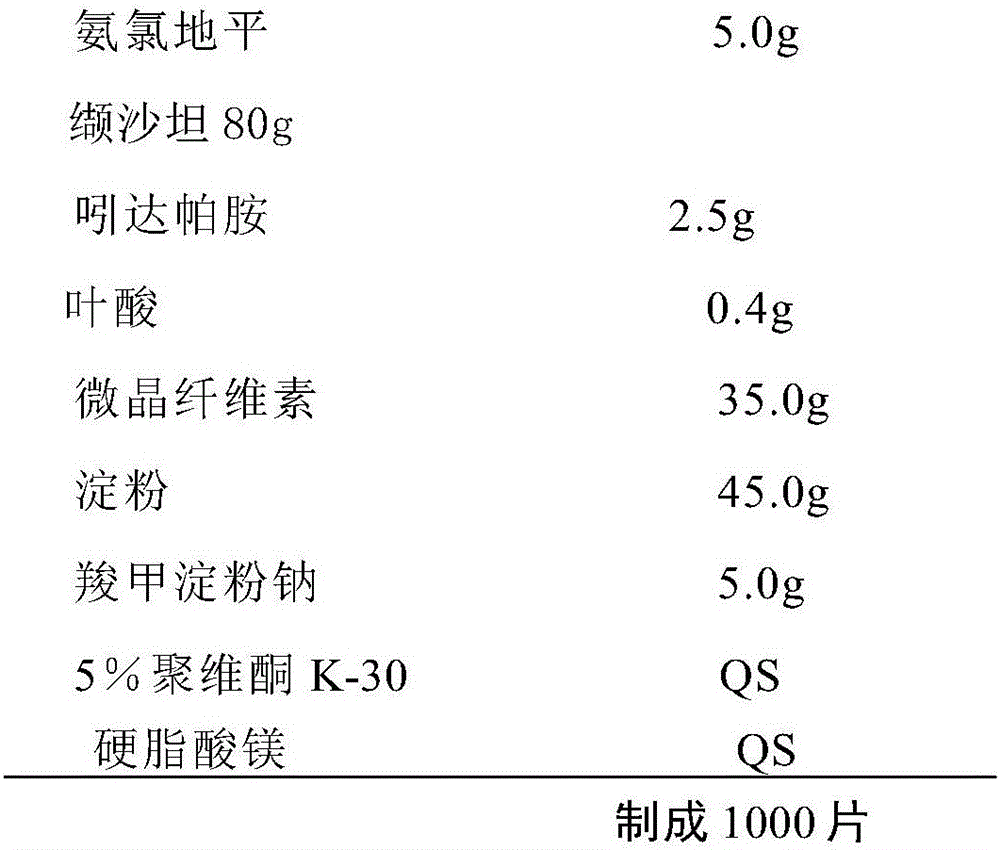
Multi-cascade antihypertensive drug composition containing folic acid
InactiveCN106310278AGood antihypertensive effectEffective protectionOrganic active ingredientsCardiovascular disorderThiazide diureticTherapeutic effect
The invention relates to a drug composition. The drug composition consists of an effective treating amount of calcium antagonist (CCB), an angiotensin II receptor blocker (ARB), a thiazide diuretic, a folic acid compound and a pharmaceutically acceptable carrier, wherein the content of the CCB is 2-240 mg, the content of the ARB is 4-600 mg, the content of the thiazide diuretic is 0.625-25 mg, and the content of the folic acid compound is 0.4-0.4 mg. The drug composition has the advantages that the drug composition can effectively improve the treatment effect of reducing blood pressure of class-2 and class-3 hypertensive patients, strengthen the effect of protecting target organs of the patients and reduce the occurrence risk of cerebral apoplexy complications caused by high blood pressure. In addition, the drug composition can be conveniently taken by patients, the treatment compliance is greatly improved, and the medical costs can be also reduced.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Antihypertensive agents
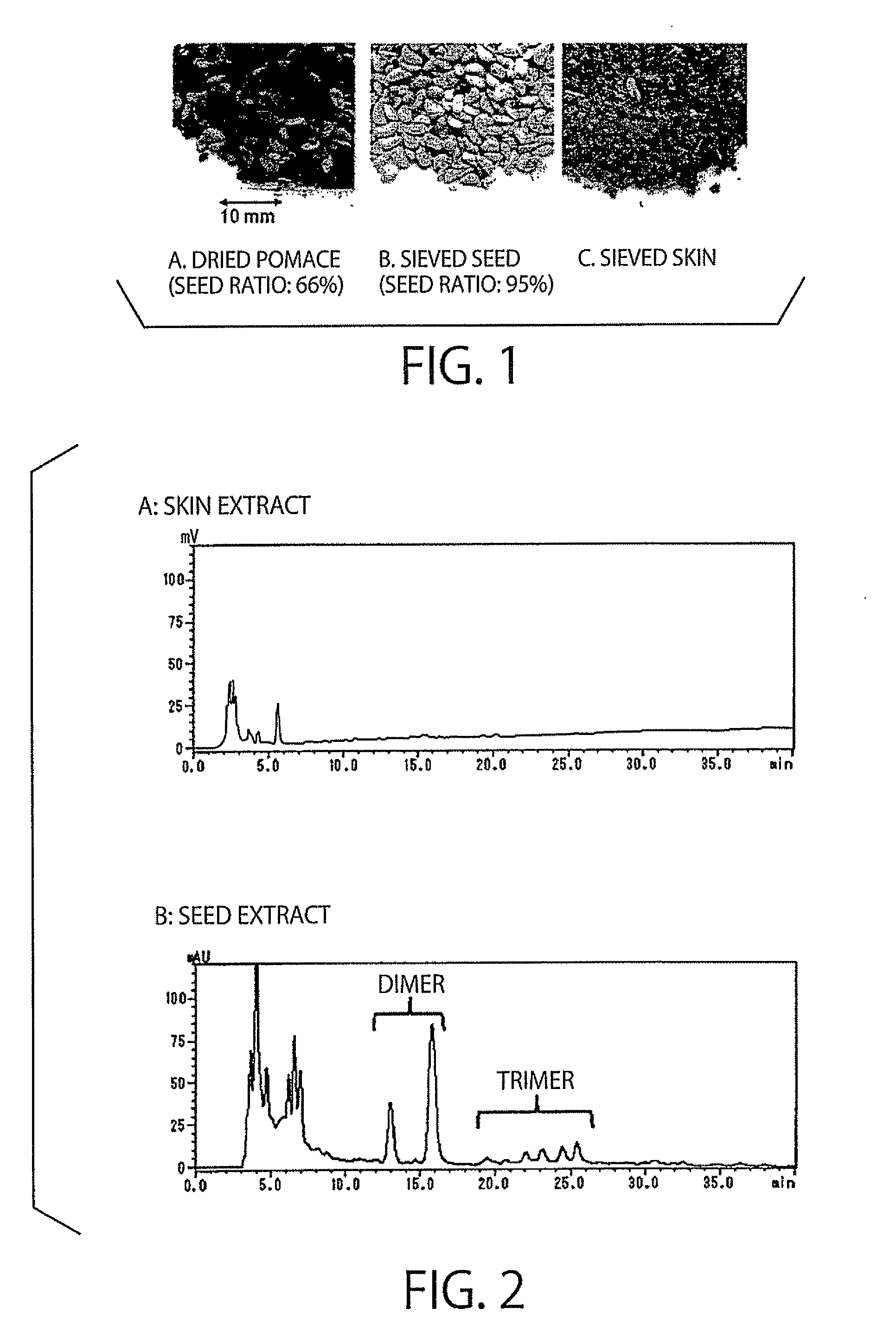
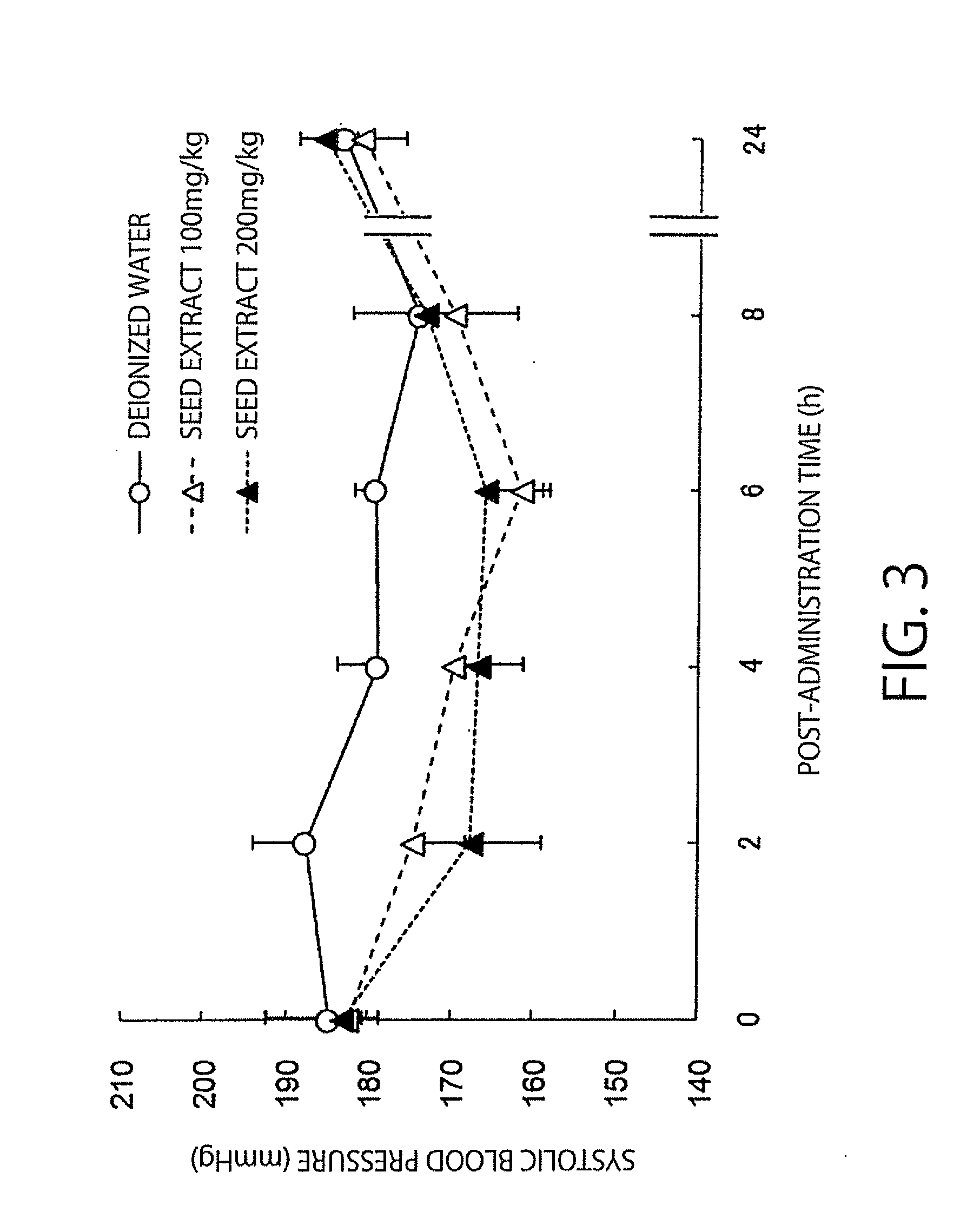
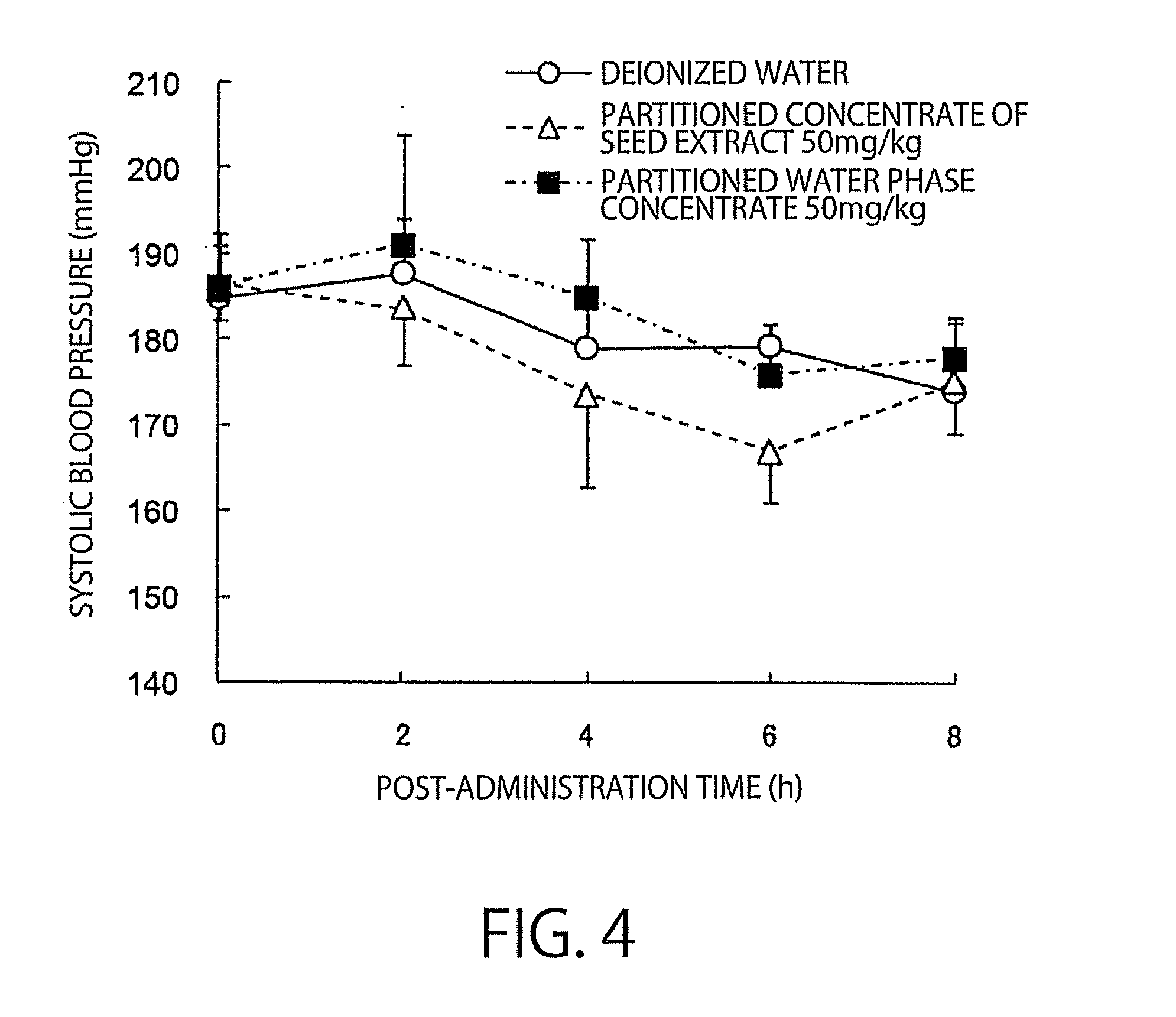
The present invention relates to an antihypertensive agent including a boysenberry seed extract as an active ingredient. According to the invention, there can be provided an effective and highly safe antihypertensive agent having antihypertensive effect so that the agent can contribute to the prevention and amelioration of hypertension and having very little risk of side effects even in continuous intake, and a method for producing the antihypertensive agent at low cost and efficiently.
Owner:BOURBON CORP
Medicinal composition contaniing angiotensin invertase inhibitor and vitamin B
ActiveCN1269529CGood curative effectReduce bleedingOrganic active ingredientsGroup 5/15 element organic compoundsAnginaCurative effect
The medicine composition consists of one of ACE1, its active metabolism product and salt in treating effective amount of 0.5-100 mg, one or several kinds one B family vitamins in treating effective amount of 0.1-50 mg, and pharmaceutically acceptable carrier. The medicine composition of the present invention has the advantages of raising the treating effect of blood pressure lowering ACE1 medicine, strengthening the protecting effect of blood pressure lowering ACE1 medicine on target organ and reducing the incidence rate of hemorrhage of the ocular fundus, angina pectoris, myocardial infarction and other complications.
Owner:SHENZHEN AUSA PHARM CO LTD
Hypoglycemic and antihypertensive medicine prepared from edible plant tissue and its preparing process and usage
InactiveCN1382483AAbundant sources of raw materialsEasy to prepareMetabolism disorderUnknown materialsGourdClinical efficacy
A hypoglycemic and antihypertensive medicine is prepared from corn tassel, carrot and white gourd peel. Its advantages are widely available raw materials, high curative effect and no by-effect.
Owner:刘卫国
Biodegradable subcutaneous implant rod used for long-acting pressure reduction
ActiveCN104147606AAvoid first pass eliminationAvoid direct stimulationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsHuman bodyGlycerol
The invention provides a biodegradable subcutaneous implant rod used for long-acting pressure reduction. The biodegradable subcutaneous implant rod is a cylindrical implant rod prepared by mixing an antihypertensive drug with a biodegradable material--modified poly(glycerol-sebacate) which is used as a carrier. The implant rod is implanted into subcutaneous tissue of the human body after cobalt 60 irradiation sterilization; through slow degradation of modified poly(glycerol-sebacate), the drug is released, enters blood circulation and performs the effect of a pharmacological antihypertensive drug, and effective plasma concentration is guaranteed so as to achieve the purposes of long-term blood pressure reduction and blood pressure stabilization. The implant rod is totally degraded in one to two years, the drug is totally released at the same time, the implant rod turns into carbon dioxide, nitrogen, water and a few amount of glycerin which are absorbed by the human body and are then discharged out from the human body, so a treatment course of slow release administration is completed. The biodegradable subcutaneous implant rod can substantially reduce the attack rate of sudden cardio-cerebrovascular emergencies due to a patient misses a dose of a drug; and according to results of clinical verification, the biodegradable subcutaneous implant rod has an effective rate of 100% on primary hypertension adaptation diseases.
Owner:北创仙途(北京)医疗技术服务有限公司
Biodegradable subcutaneous implant sticks for long-term blood pressure reduction
ActiveCN104147606BAvoid first pass eliminationAvoid direct stimulationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsHuman bodyGlycerol
The invention provides a biodegradable subcutaneous implant rod used for long-acting pressure reduction. The biodegradable subcutaneous implant rod is a cylindrical implant rod prepared by mixing an antihypertensive drug with a biodegradable material--modified poly(glycerol-sebacate) which is used as a carrier. The implant rod is implanted into subcutaneous tissue of the human body after cobalt 60 irradiation sterilization; through slow degradation of modified poly(glycerol-sebacate), the drug is released, enters blood circulation and performs the effect of a pharmacological antihypertensive drug, and effective plasma concentration is guaranteed so as to achieve the purposes of long-term blood pressure reduction and blood pressure stabilization. The implant rod is totally degraded in one to two years, the drug is totally released at the same time, the implant rod turns into carbon dioxide, nitrogen, water and a few amount of glycerin which are absorbed by the human body and are then discharged out from the human body, so a treatment course of slow release administration is completed. The biodegradable subcutaneous implant rod can substantially reduce the attack rate of sudden cardio-cerebrovascular emergencies due to a patient misses a dose of a drug; and according to results of clinical verification, the biodegradable subcutaneous implant rod has an effective rate of 100% on primary hypertension adaptation diseases.
Owner:吕汇川
Medicated pillow for reducing high blood pressure and manufacturing method thereof
InactiveCN1454557ATo achieve the purpose of treating high blood pressurePillowsUnknown materialsHerb medicineMedicine
The invention is a medicine pillow for reducing one's blood pressure, made by composed of special herbal medicine produced in Guizhou and composed of pillowslip and pillow core. The pillow core consists of pillow cushion and medicine bag, the pillow crushion able to be made by animal pair, or cotton fibre, or synthetic fiber or other plant fiber and the medicine bag packing with: celery root 150-200 shares (quality, same to the following), carpinus turcazninowii 100-150 shares, cogongrass rhizome 100-150 shares, mother chrysanthemum 100-150 shares and gypsum powder 500 shares. It can be widely used in treatment and health cure of the hypertension.
Owner:刘兴中
Antihypertensive medical composite
InactiveCN101869710AGood curative effectImprove protectionMetabolism disorderUrinary disorderHypotensive drugNK1 receptor antagonist
The invention relates to an antihypertensive medical composite which is composed of 0.5-100mg of angiotensin converting enzyme inhibitor with curative dose, 4-800mg of AT1 receptor antagonist, 0.1-50mg of one or more of B-complex vitamins and pharmaceutically acceptable carrier in one tablet. The invention has the advantages that the medical composite can increase the curative effect of the antihypertensive medicine, improve the target organ protection function of the medicine and reduce the incidence rate of complications such as fundus hemorrhage, angina pectoris, myocardial infarction, brain stroke, heart failure and kidney failure. In addition, the antihypertensive medical composite can be used to ensure the patient to take the medicine conveniently and reduce medical expenses.
Owner:北京奥萨医药研究中心有限公司 +1
Antihypertensive pharmaceutical composition as well as preparation method and application thereof
InactiveCN112741832ADefinite curative effectSmall toxicityOrganic active ingredientsCardiovascular disorderSide effectMedicine
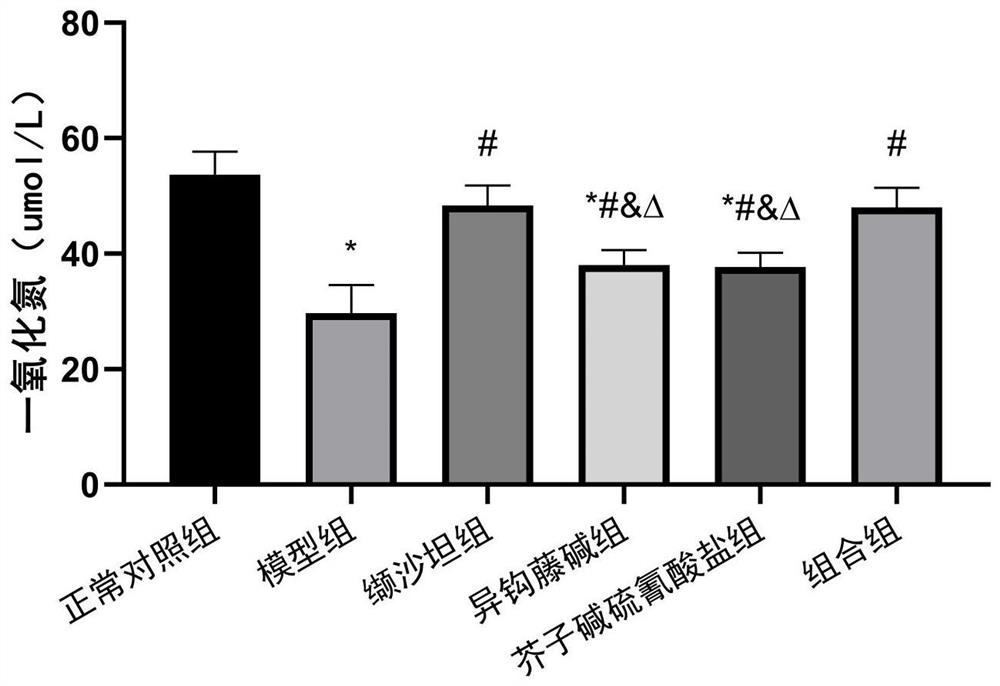
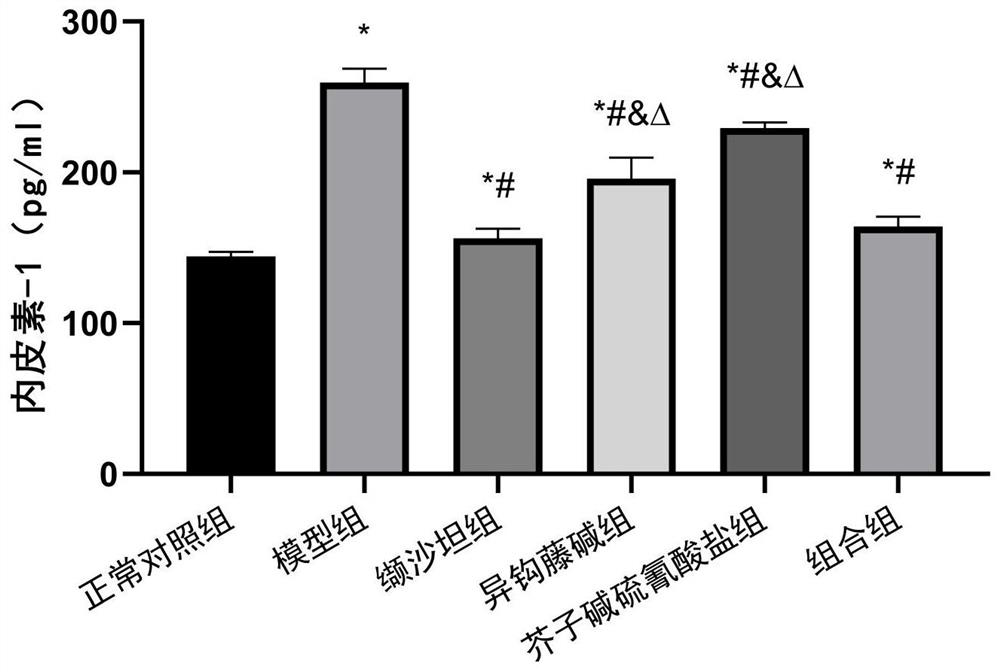
The invention relates to an antihypertensive pharmaceutical composition as well as a preparation method and application thereof, and belongs to the technical field of antihypertensive drugs. The pharmaceutical composition disclosed by the invention is prepared from isorhynchophylline and sinapine thiocyanate. The pharmaceutical composition is low in price, easy to obtain, definite in curative effect, low in toxic and side effect and capable of preventing and treating hypertension in a multi-target mode.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
Bloos-pressure-reducing health-care food and preparation method thereof
The invention relates to a blood-pressure-reducing nourishing food. Usually adverse influences can be caused on other human functions when an anti-hypertensive drug is used for a long time. The product provided by the invention has no side effect to human body, and provides a nourishing effect when used for a long time. The food has both blood-pressure-reducing and life-preserving effects. The food is prepared from the materials of honey, rock candy, purple sweet potato, orange peel, pear, bitter gourd, oats, yam, wax gourd, red date, hawthorn fruit and glutinous rice. The materials are easy to obtain, and operation is easy.
Owner:陈永宏
Medicinal composition containing calcium passage paralysor and B family vitamin and its use
ActiveCN100551442CImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderDiseasePatient compliance
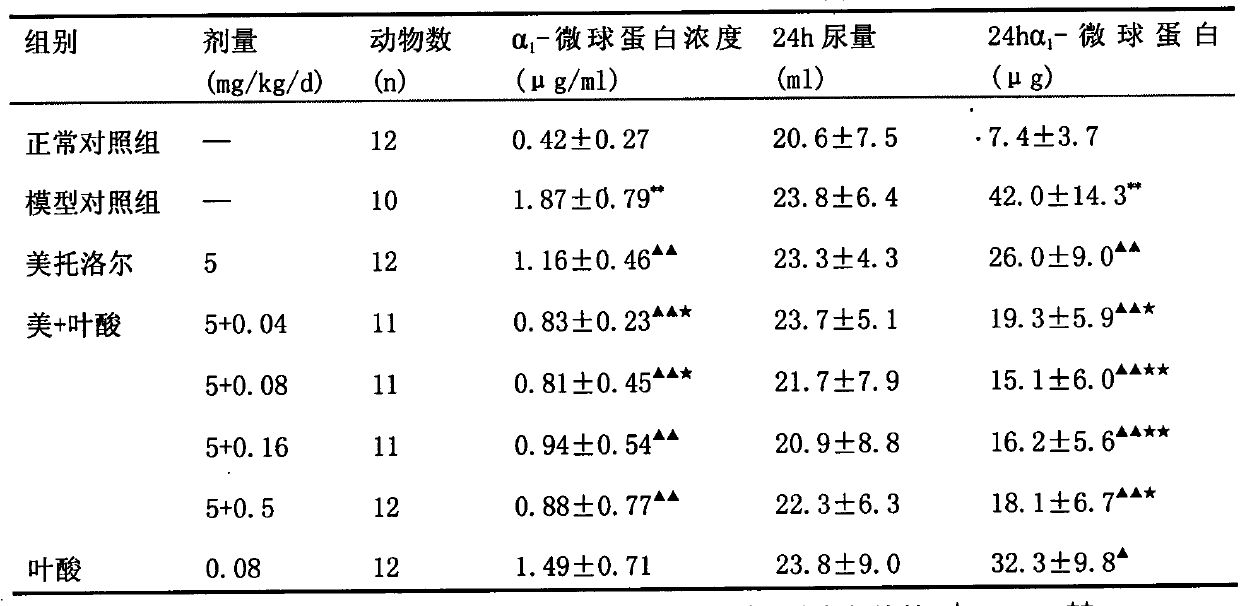
The invention relates to a pharmaceutical composition containing a calcium channel blocker and B vitamins. One or more of B vitamins and a pharmaceutically acceptable carrier are used. The pharmaceutical composition can improve the curative effect of calcium channel blockers and antihypertensive drugs, enhance the protective effect of calcium channel blockers on target organs, and reduce complications such as fundus hemorrhage, angina pectoris, myocardial infarction, cerebral apoplexy, heart failure, and renal failure. incidence of disease. Also provided is the application of the pharmaceutical composition in the preparation of medicines for preventing, treating and delaying hypertension complicated with hyperlipidemia. Through the implementation of the present invention, the pharmaceutical composition provided to patients not only has reliable curative effect, but also improves patient compliance, makes it convenient for patients to take medicine, reduces medical expenses, and has a good market prospect.
Owner:SHENZHEN AUSA PHARMA +1
Compound medicine for reducing blood pressure and blood fat and preparation method thereof
InactiveCN102671198AEffective treatmentGood treatment effectMetabolism disorderPill deliveryBlood pressure kitPatient compliance
The invention relates to the technical field of biomedicines, in particular to a compound medicine for reducing blood pressure and blood fat and a preparation method thereof. The compound medicine for reducing blood pressure and blood fat consists of a thiazine hypotensor and a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, and can simultaneously treat hypertension and hyperlipidemia; the hypotensor and a hypolipidemic are not required to be taken simultaneously; the compound medicine is used for reducing blood pressure and blood fat by synergistic effect of the thiazine hypotensor and the HMG-CoA reductase inhibitor, improves compliance of patients and reduces adverse reaction; the compound medicine has a good therapeutic effect on hypertension and hyperlipidemia in clinical trials; and moreover, the invention also provides the preparation method thereof, technical bottleneck problems are solved, the thiazine hypotensor and the HMG-CoA reductase inhibitor can be stably used in the compound medicine, respective properties of the thiazine hypotensor and the HMG-CoA reductase inhibitor are not affected, and patients suffering from hypertension and hyperlipidemia are simultaneously effectively treated.
Owner:FOSHAN DAYI TECH LTD
Beta-adrenergic receptor retarder and vitamins B-containing pharmaceutical composition and applications thereof
ActiveCN103272236ALittle side effectsBlood pressure controlOrganic active ingredientsSenses disorderBeta blockerCoronary heart disease
The invention relates to a beta-adrenergic receptor retarder (short for beta receptor retarder) and vitamins B-containing pharmaceutical composition which comprises effective treatment quantity of one of beta receptor retarder and an active metabolite or salt thereof, effective treatment quantity of one or more of vitamins B, and a pharmaceutically acceptable carrier, wherein the content of the beta receptor retarder and the active metabolite or salt thereof is 2.5-200mg, and the content of vitamins B is 0.1-50mg. The beta-adrenergic receptor retarder and vitamins B-containing pharmaceutical composition has the advantages that with the pharmaceutical composition, the curative effect of the beta receptor retarder on reducing the blood pressure can be enhanced, the target organ protection role of the beta receptor retarder hypotensive drugs can be enhanced, and the occurrence rate of complications such as left ventricular hypertrophy, coronary heart disease, heart failure, cerebral apoplexy, renal lesions, renal insufficiency and the like can be reduced. In addition, the pharmaceutical composition can enable patients to take conveniently, and the medical cost can be lowered.
Owner:SHENZHEN AUSA PHARM CO LTD +1
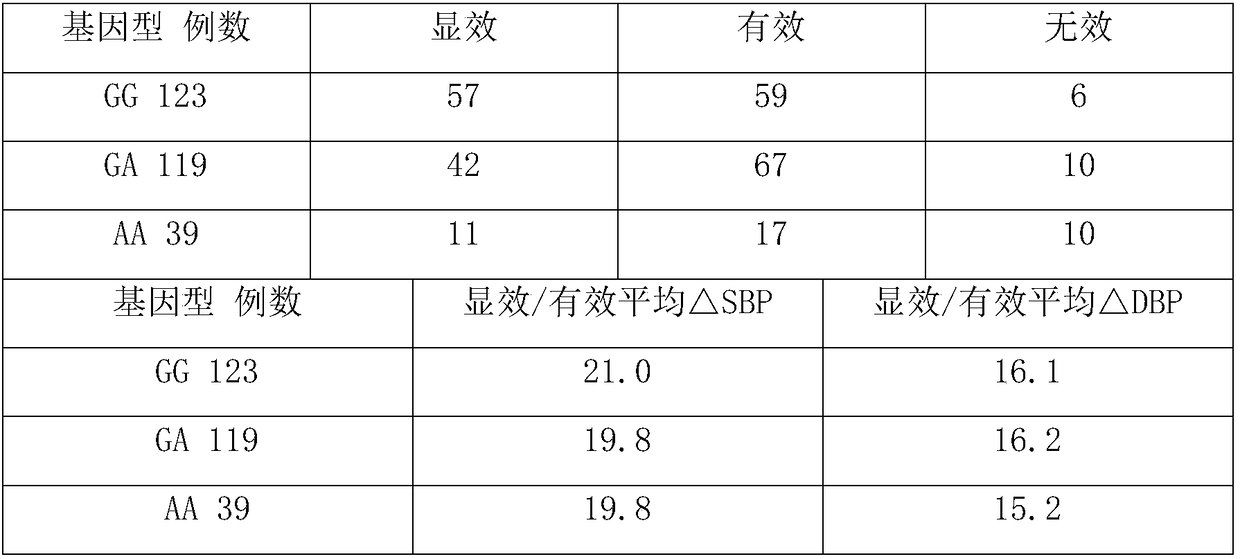
Establishment of methodology for detecting genes affecting efficacy of antihypertensive drugs by TaqMan-MGB probe technique
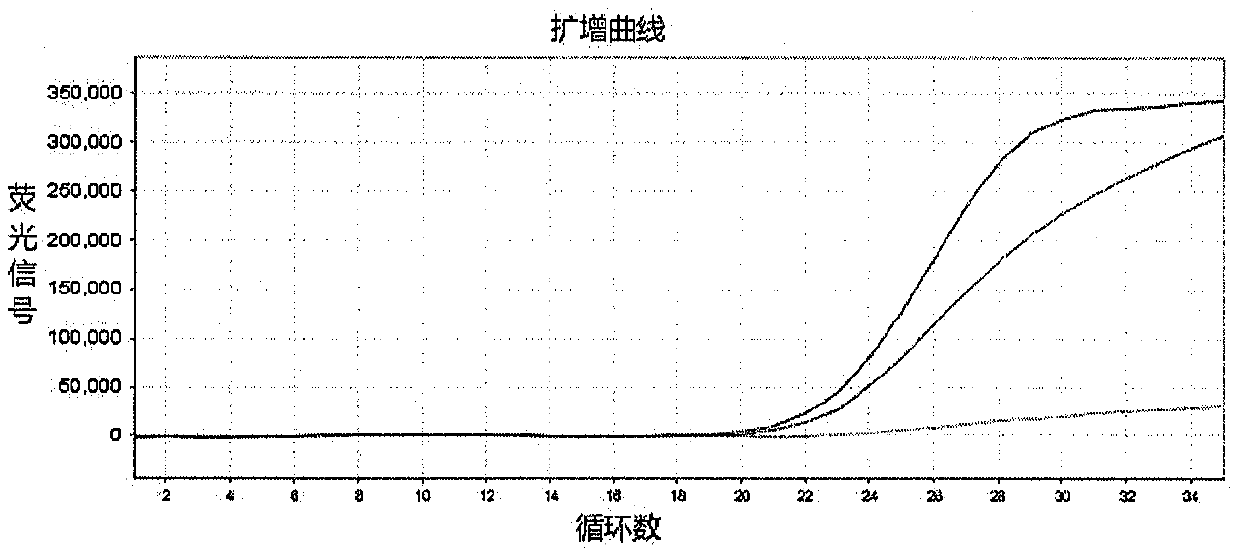
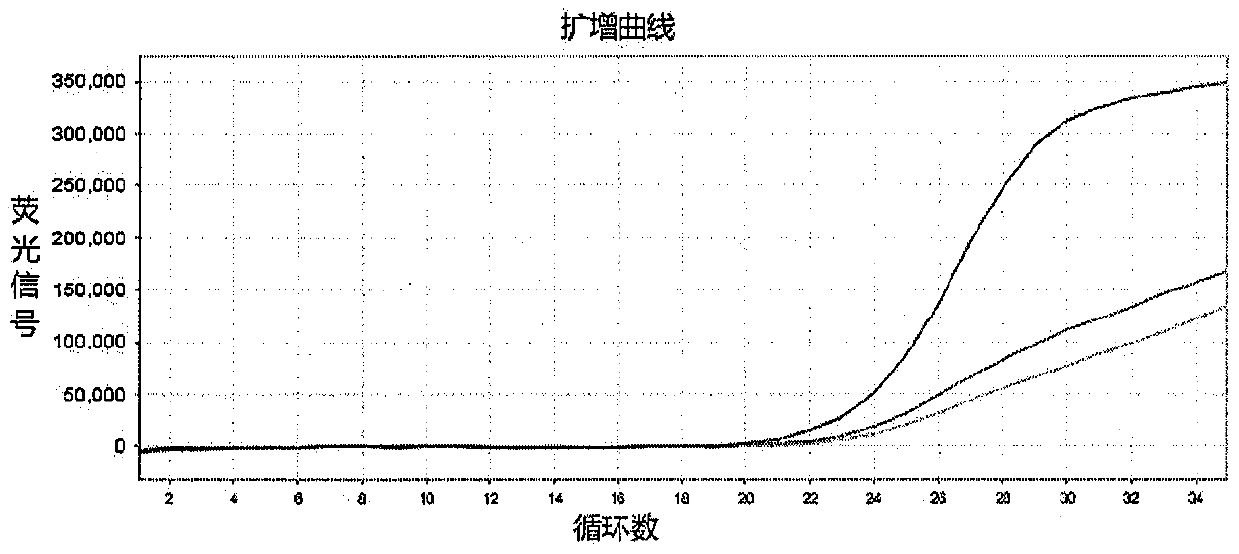
InactiveCN109897895AQuick checkEasy to readMicrobiological testing/measurementDNA/RNA fragmentationDrug interactionFluorescence
The invention discloses a method for detecting genes affecting the efficacy of antihypertensive drugs by combining a TaqMan-MGB probe and a real-time fluorescence quantitative PCR technique. The polymorphism of the genes affecting the efficacy of the antihypertensive drugs in samples to be detected is detected, five SNP loci can be detected simultaneously, used primers and the TaqMan-MGB probe have high specificity, the risk of false positive caused by PCR product contamination can be reduced, the operation is simple and the result is accurate and reliable. According to the method, functionalmeaningful mutations such as NEDD4L-326G>A(rs4149601), AGTR1 1186A>C(rs5186), ACE I / D(rs1799752), ADRB1 1165G>C(rs1801253) and CYP3A5*3(rs776746) are detected to assist clinicians to select appropriate drugs and doses or avoid occurring of drug interactions, the best therapeutic effect for patients is achieved, and thus the purpose of real ''individualized medication'' is achieved.
Owner:江苏百世诺医疗科技有限公司
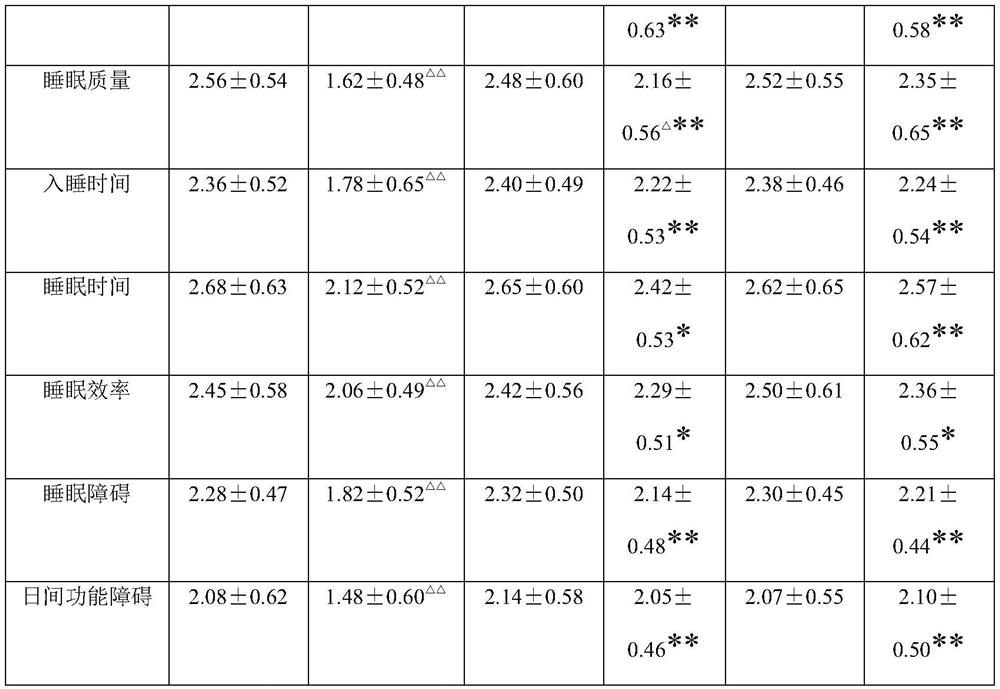
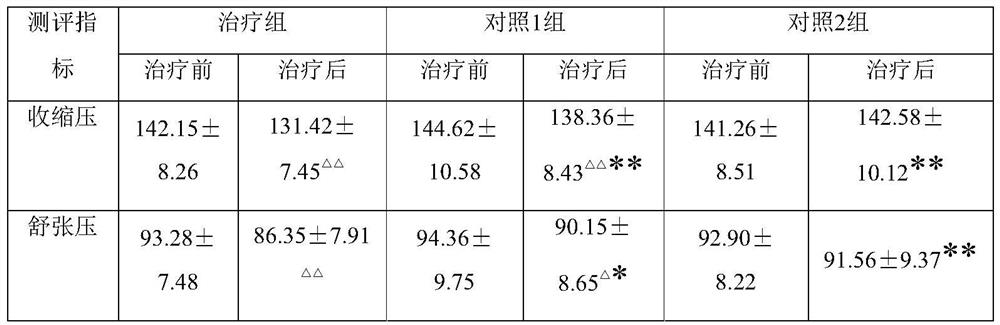
A traditional Chinese medicine composition for treating hypertensive insomnia
ActiveCN107875360BImprove the quality of lifeImprove sleepingNervous disorderCardiovascular disorderHypertension mildAstragalus mongholicus
The invention provides a traditional Chinese medicine composition for treating hypertension insomnia, which is prepared from 10-20 parts of codonopsis pilosula, 10-20 parts of argehead atractylodes rhizom, 15-25 parts of astragalus membranaceus, 15-25 parts of dogwood, 10-20 parts of schisandra chinensis, 5-15 parts of cimicifugae foetidae, 5-15 parts of rhizoma zingiberis, 5-15 parts of cinnamon,2-10 parts of radix bupleuri and 2-10 parts of honey-fried liquorice root. The traditional Chinese medicine composition can reduce the systolic pressure and diastolic pressure levels of patients suffering from moderate hypertension and mild hypertension, and can effectively improve the sleeping state, improve the sleep quality and improve the quality of life of patients with hypertension. The invention also provides a preparation method of the traditional Chinese medicine composition for treating hypertension insomnia, and according to the preparation method, the traditional Chinese medicinecomposition can be prepared by heating and extracting the materials by taking water or ethanol with concentration of 50-95% as a solvent.
Owner:李影
Composition containing biguanide antidiabetic medicament and ACEI (Angiotensin-Converting Enzyme Inhibitors) medicament and applications thereof
InactiveCN101869572ASignificantly effective in treating diabetes combined with hypertensionCombined prevention or treatment of hypertensionMetabolism disorderHeterocyclic compound active ingredientsAngiotensin-converting enzymeDiabetes mellitus
The invention relates to a medicament composition containing biguanide antidiabetic medicament, ACEI (Angiotensin-Converting Enzyme Inhibitors) medicament and a pharmaceutically acceptable carrier. The invention also relates to the applications of the medicament composition in the preparation of medicaments for preventing or treating diabetes mellitus with hypertension. The implementation of the invention provides the medicament composition with specific applications, and the medicament composition not only has a precise curative effect, but also can improve the compliance of patients. The patients can take the medicament conveniently, and the medical expense is reduced. The invention has a better market prospect, and belongs to the field of pharmacy.
Owner:北京奥萨医药研究中心有限公司 +1
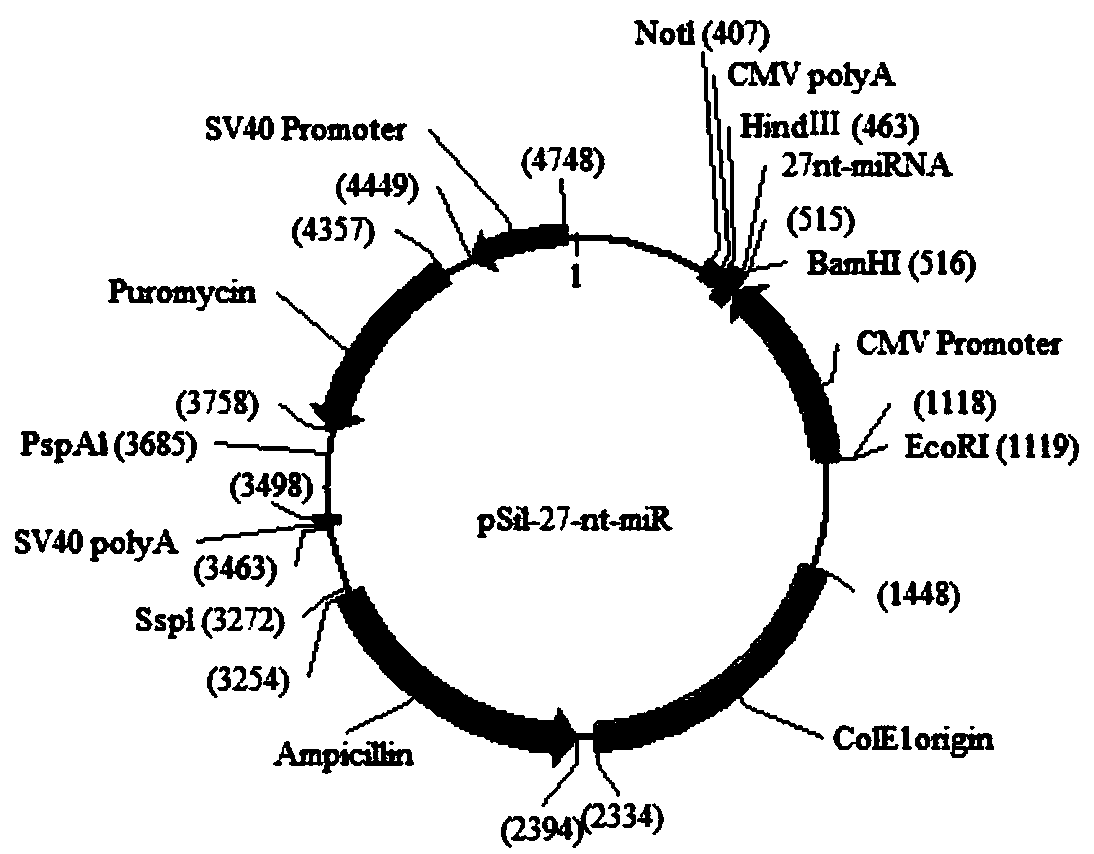
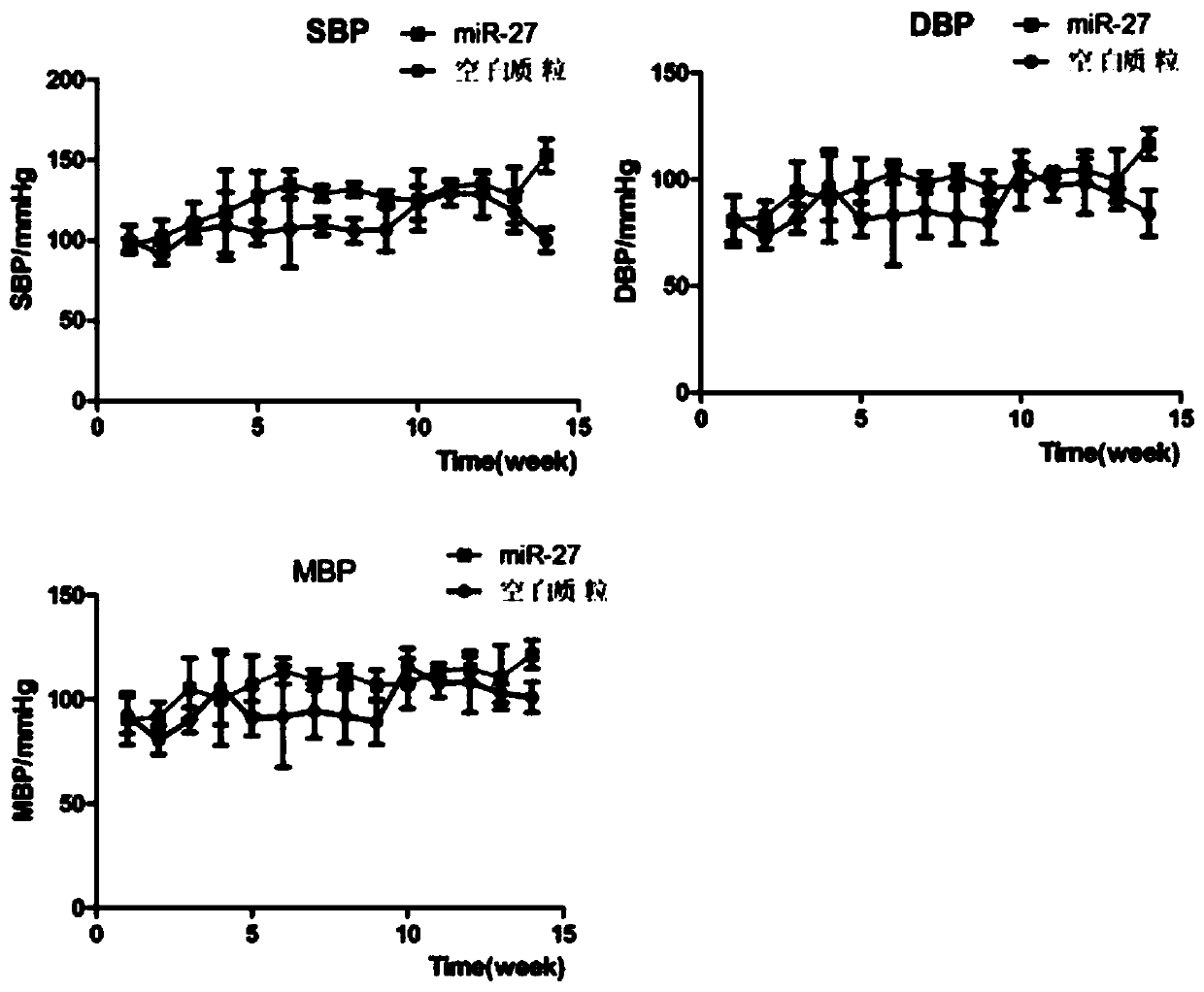
Human intron derived 27 base microRNA and application thereof to blood pressure regulation
The invention discloses a human intron derived 27 base microRNA and an application thereof to blood pressure regulation. The human intron derived 27 base microRNA is generated by four-time duplicationof a fourth intron 27 base duplicon of eNOS (endothelial nitric oxide synthase), and the nucleotide sequence of the human intron derived 27 base microRNA is as shown in SEQ ID NO. 1. A high-expression plasmid is prepared according to the sequence, the high-expression plasmid and lipidosome are mixed according to the weight ratio of 3:1 and diluted by normal saline, and mixture is injected into the body of an SD (sprague-daw) rat through rat caudal veins to prove that the human intron derived 27 base microRNA has a regulating effect on rat blood pressure, can be used for researching hypertension diseases, finally can be used for screening some antihypertensive drugs and preparing a hypertension early warning chip and has a potential application prospect.
Owner:GUANGXI INT ZHUANG MEDICINE HOSPITAL
Method for micronization of raw materials of dihydropyridine antihypertensive drugs
The application provides a new method for micronizing dihydropyridine antihypertensive drug raw materials, which relates to the technical field of biopharmaceutical preparations. A new method for micronizing dihydropyridine antihypertensive drug raw materials, comprising: dissolving the dihydropyridine antihypertensive drug raw materials in a first solvent, dissolving the first dihydropyridine antihypertensive drug raw materials The solvent is added to the second solvent to realize anti-solvent recrystallization of dihydropyridine antihypertensive drugs; the first solvent includes lower aliphatic alcohols or aliphatic ketones; the second solvent includes water, an aqueous solution containing electrolytes, or surfactants aqueous solution. The method can further improve the in vivo bioavailability of the oral preparations of felodipine and other dihydropyridine antihypertensive drugs.
Owner:HEFEI LIFEON PHARMA
Composition of polydextrose and calcium ion antagonist and application thereof
InactiveCN110179815ABest combination ratioAddresses constipation side effectsOrganic active ingredientsDigestive systemIntestinal structureSide effect
The invention relates to a composition of polydextrose and a calcium ion antagonist, which can reduce the side effect of constipation caused by taking antihypertensive drugs by using polydextrose which is not absorbed by the intestine while reducing blood pressure by the calcium ion antagonist, and belongs to the technical field of compositions of polydextrose and a calcium ion antagonist and application thereof. According to the composition of polydextrose and a calcium ion antagonist, the mass ratio of the polydextrose to the calcium ion antagonist is from (20:1) to (5:1). The composition ofthe polydextrose and a calcium ion antagonist of the invention can solve the side effect of constipation caused by long-term use of the calcium ion antagonist, the latest action mechanism of the action is revealed, and the optimal combination ratio of the polydextrose and the calcium ion antagonist is provided; good application prospects are obtained.
Owner:LUDONG UNIVERSITY
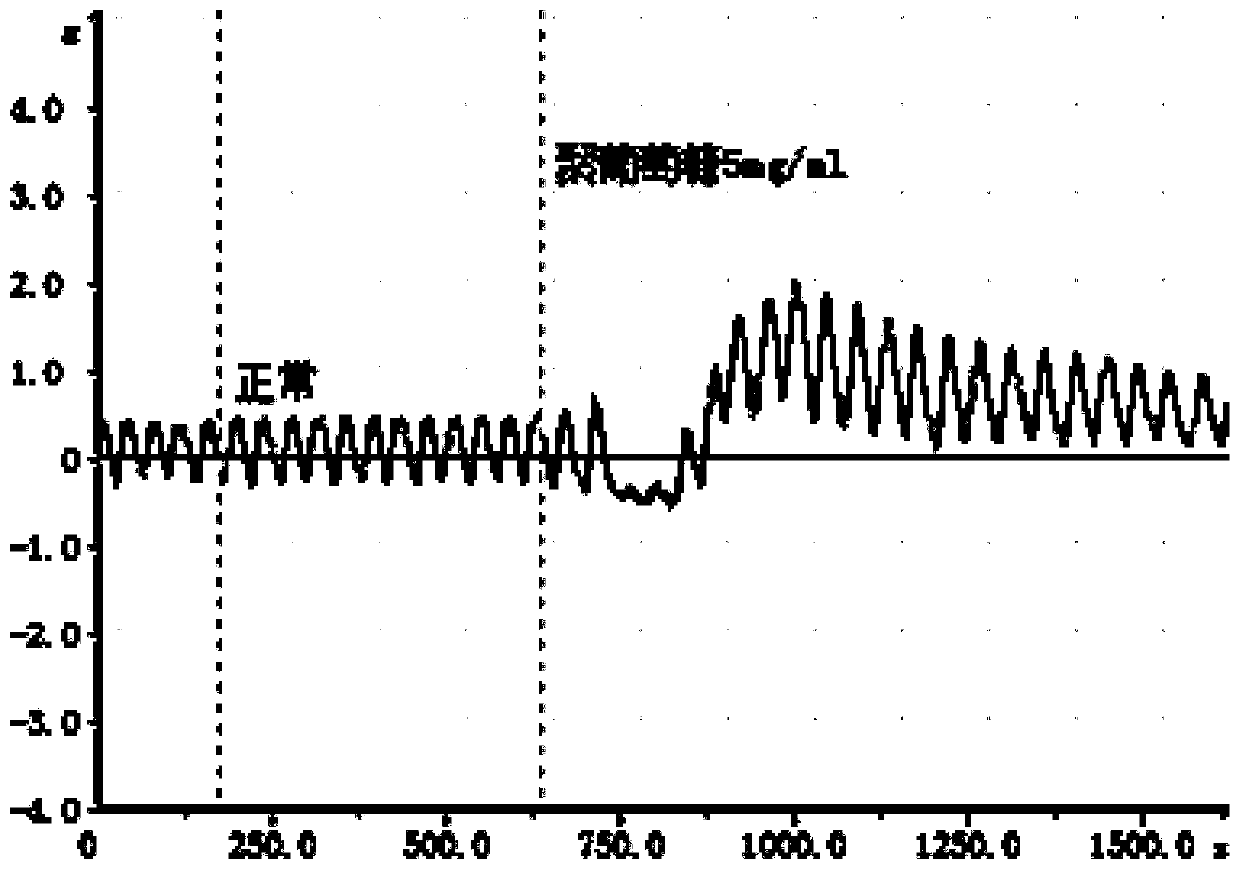
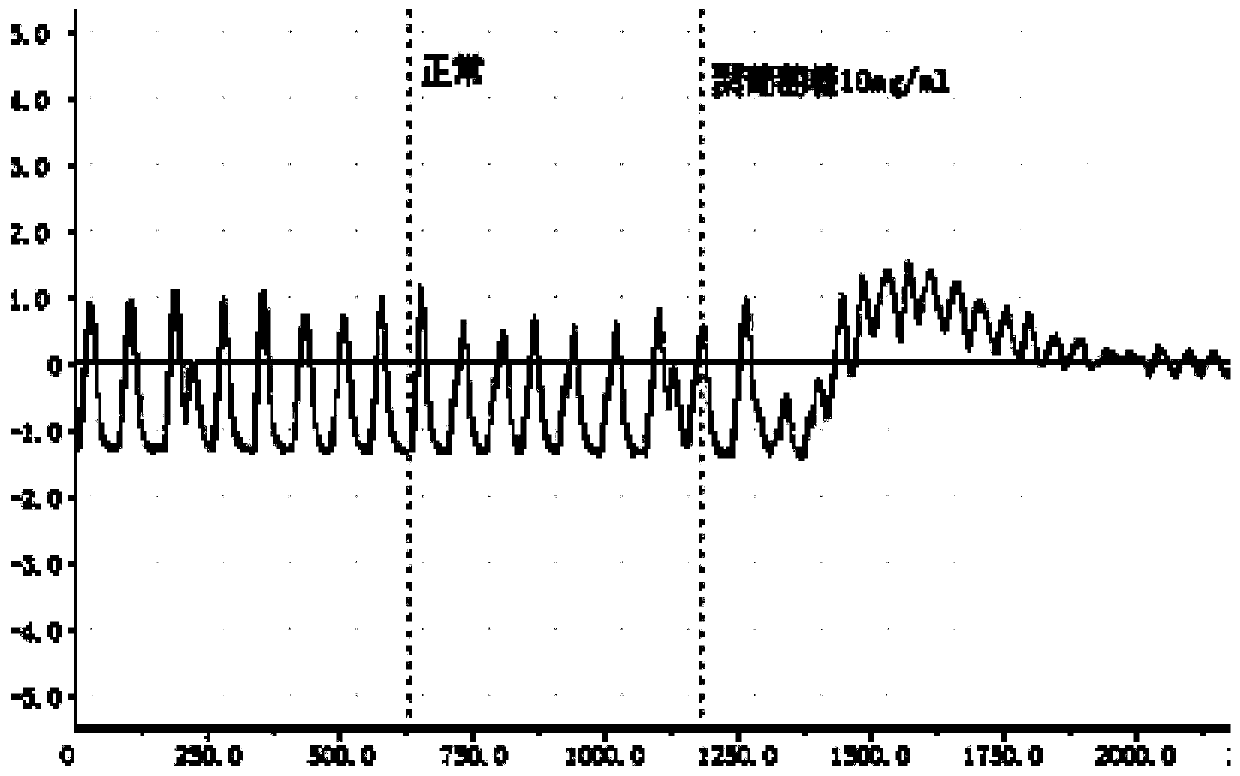
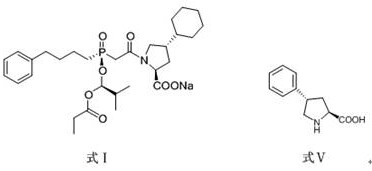
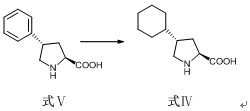
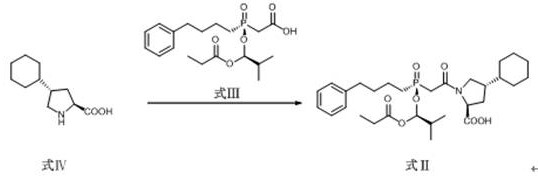
A preparation method of antihypertensive drug fosinopril sodium and its key intermediate
ActiveCN107365268BOrganic chemistry methodsGroup 5/15 element organic compoundsChiral selectivityAntihypertensive medication
The present invention relates to a kind of antihypertensive drug fosinopril sodium suitable for industrialized production and the preparation method of its key intermediate trans-4-phenyl-L-proline, wherein the key intermediate trans-4-phenyl The synthesis of base-L-proline has high chiral selectivity, and the method is simple and easy to operate.
Owner:CHANGZHOU PHARMA FACTORY
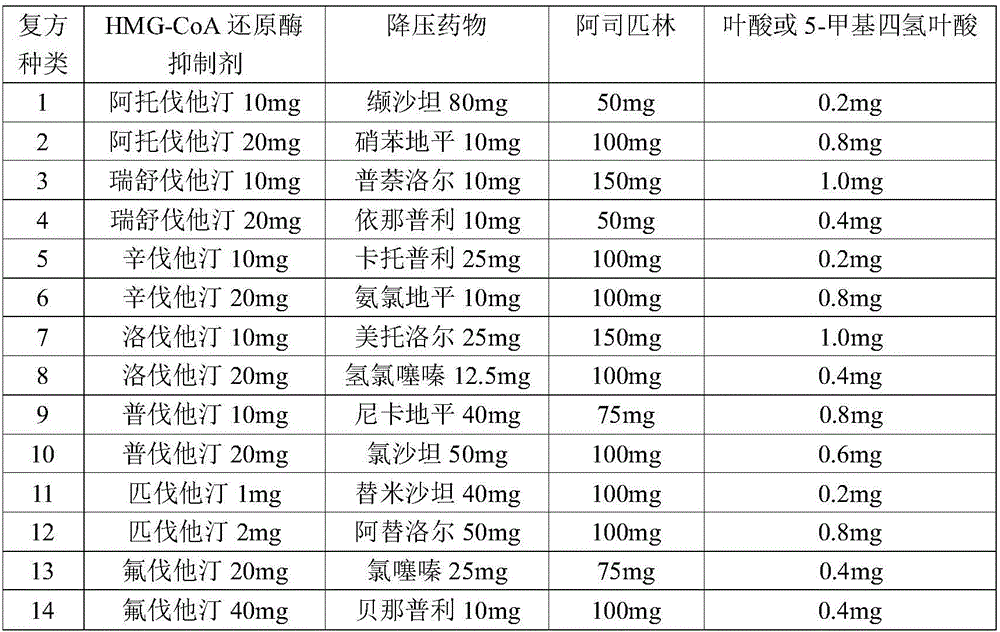
Pharmaceutical composition for preventing cardiovascular diseases
InactiveCN106310274AWith blood pressure loweringWith hypolipidemicSenses disorderUrinary disorderHMG-CoA reductasePatient compliance
The invention relates to a synergistic antihypertensive lipid-lowering pharmaceutical composition and the use of the pharmaceutical composition. The pharmaceutical composition comprises a pharmaceutical dosage of one of HMG-CoA reductase inhibitors, a pharmaceutical dosage of one of antihypertensive drugs, aspirin, folic acid and 5-methylaretetrahydrofolate, and a pharmaceutically acceptable carrier. The indication is hypertension accompanied with hyperlipidemia. The pharmaceutical composition can also be used as a primary drug for prevention of cardiovascular diseases. By the implementation of the invention, the pharmaceutical composition for the specific use for patients can improve the compliance of the patients, facilitate administration of the patients and reduce medical expenses, so that the pharmaceutical composition has relatively good market prospects.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Medicinal composition containing angiotensin II receptor antagonist and vitamin B
ActiveCN1286844CGood curative effectImprove protectionSenses disorderMetabolism disorderAnginaNK1 receptor antagonist
The medicine composition consists of one of AT1 receptor antagonist, its active metabolism product, ester and salt in treating effective amount of 4-800 mg, one or several kinds one B family vitamins in treating effective amount of 0.1-50 mg, and pharmaceutically acceptable carrier. The medicine composition of the present invention has the beneficial effects of raising the treating effect of blood pressure lowering AT1 receptor antagonist, strengthening the protecting effect of blood pressure lowering AT1 receptor antagonist on target organ and reducing the incidence rate of hemorrhage of the ocular fundus, angina pectoris, myocardial infarction and other complications.
Owner:SHENZHEN AUSA PHARM CO LTD +2
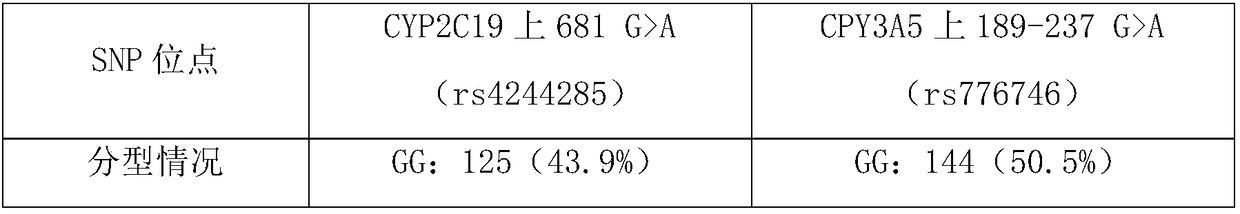
Application and kit of gene SNP locus used in hypertension individual medicine treatment genetype detection
The present application belongs to the field of molecular biological detection and cardiovascular and cerebrovascular diseases, specifically relates to application and kit of gene SNP locus used in hypertension individual medicine treatment genetype detection. According to the invention, a reagent for detecting SNP site genotypes on CYP2C19 and CPY3A5 is provided for use in the preparation of a kit for predicting telmisartan antihypertensive efficacy, and a combination of the corresponding kit and SNP site. A corresponding kit and SNP site combination is also provide.
Owner:NINGBO AJCORE BIOSCIENCES INC
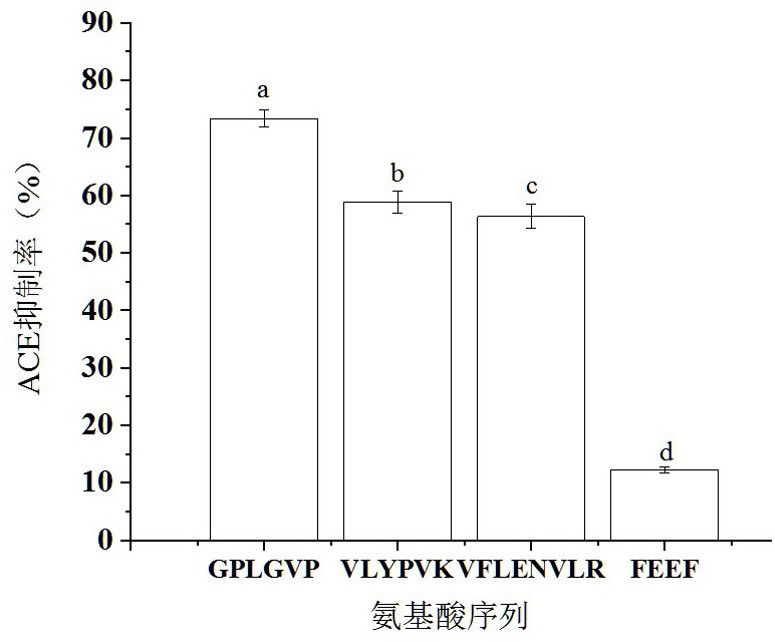
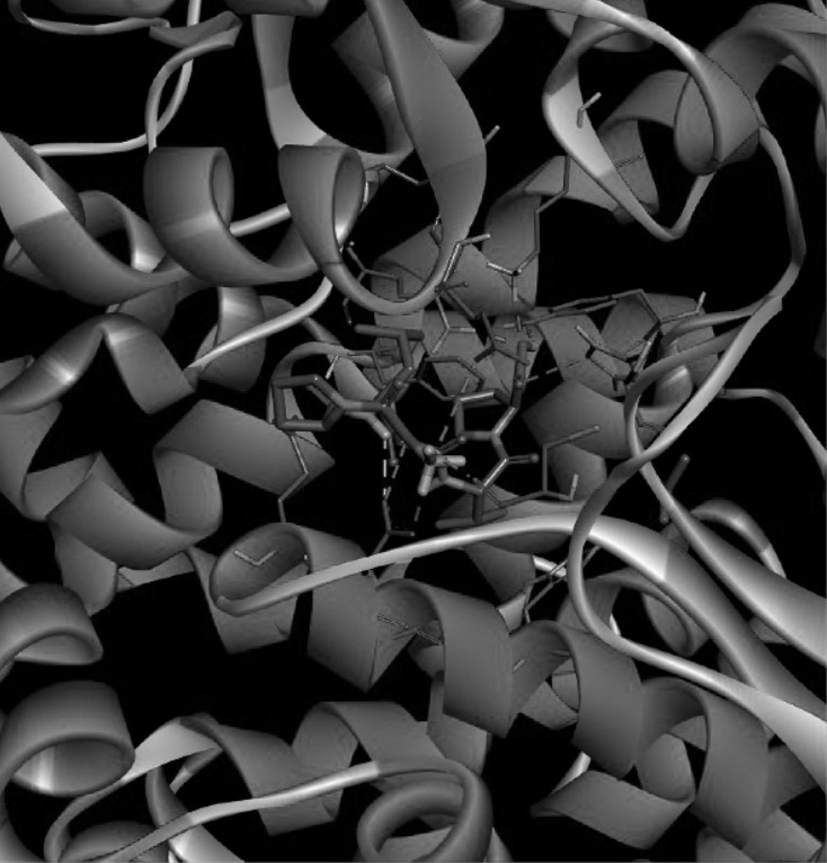
ACE inhibitory peptide as well as synthesis method and application thereof
InactiveCN113292637AHigh activityHigh purityPeptide/protein ingredientsPeptide preparation methodsAntihypertensive medicationOrganic chemistry
The invention discloses an ACE inhibitory peptide and a synthesis method and application thereof. The ACE inhibitory peptide is artificially synthesized by means of an Aapptec396 automatic peptide synthesizer and by means of an Fmoc solid-phase synthesis method, the amino acid sequence of the ACE inhibitory peptide is Gly-Pro-Leu-Gly-Val-Pro, and it is found through detection that the IC50 value of the ACE inhibitory peptide is 105.8 mu M (P is smaller than 0.05), so that the ACE inhibitory peptide is a better-activity ACE inhibitor and can be used for preparing antihypertensive drugs.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI +1
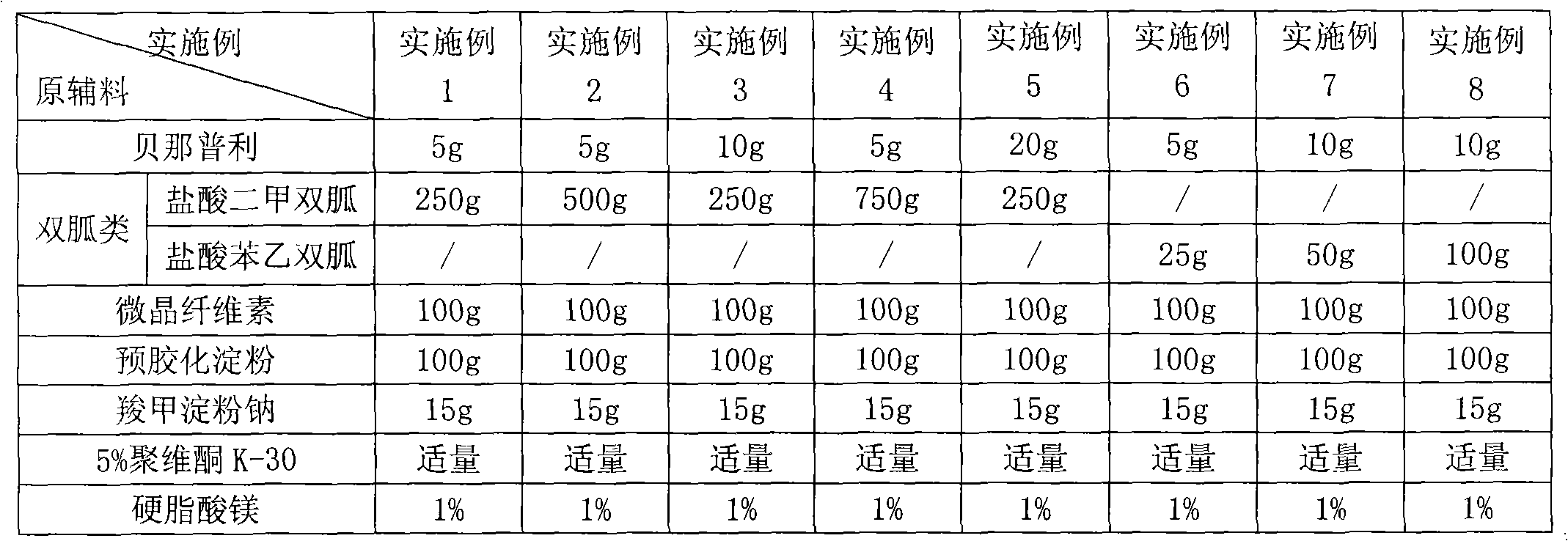
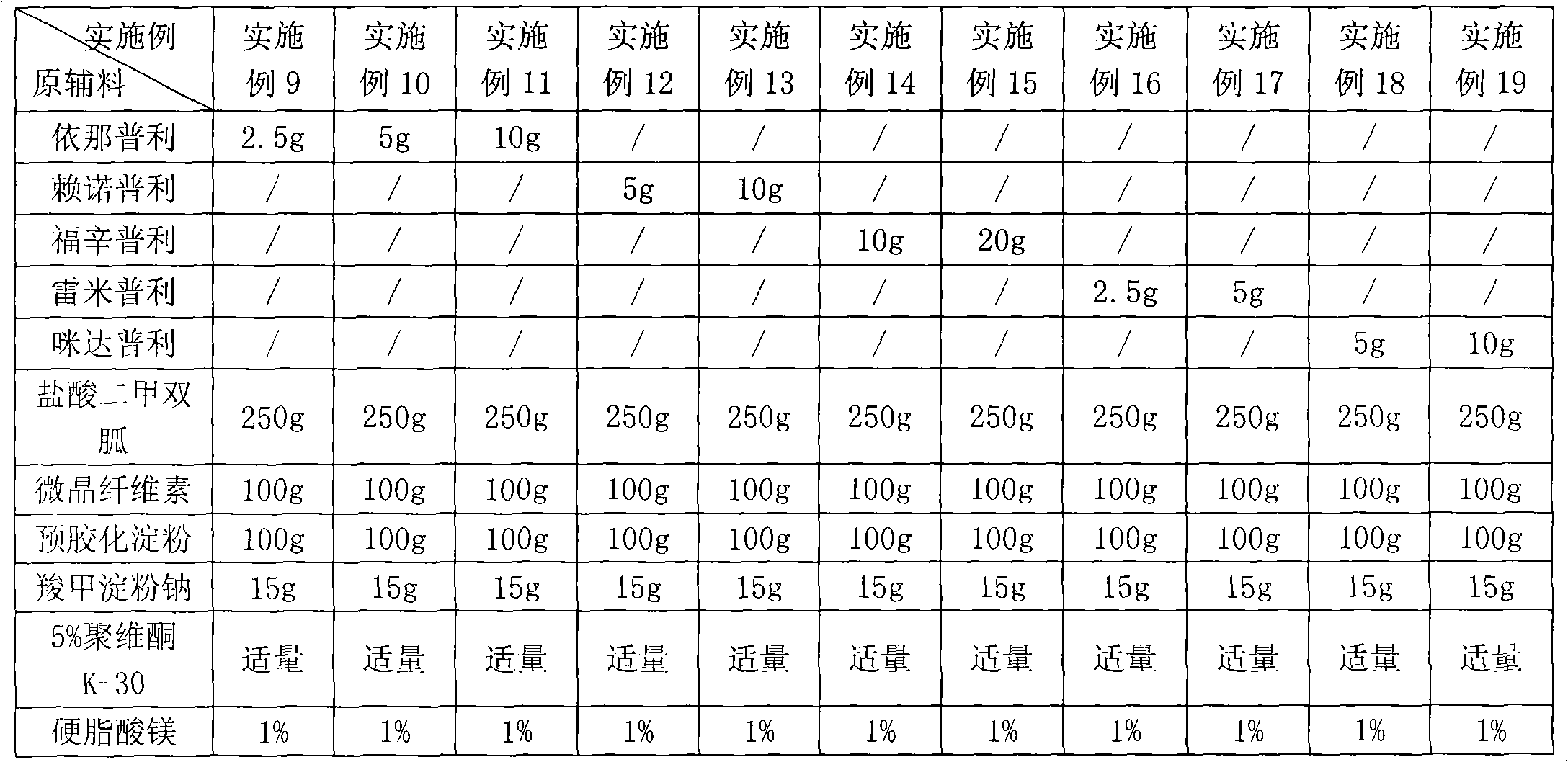
Compound antihypertensive pharmaceutical composition and preparation method thereof
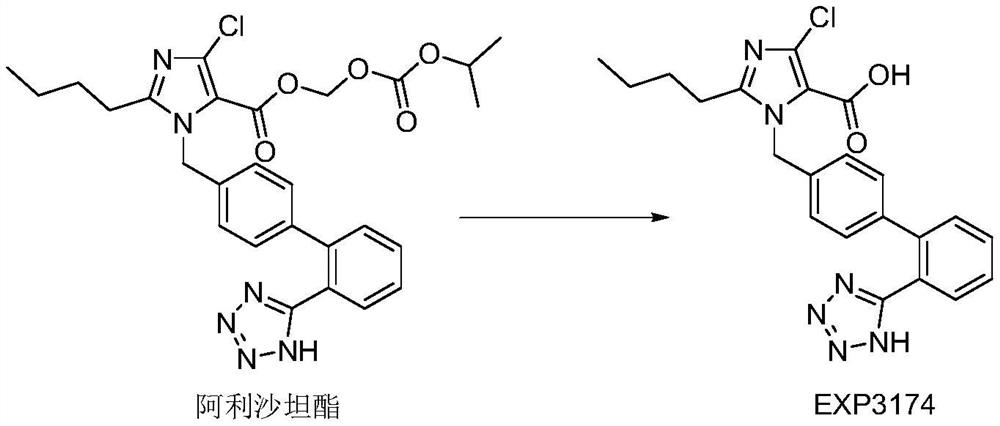
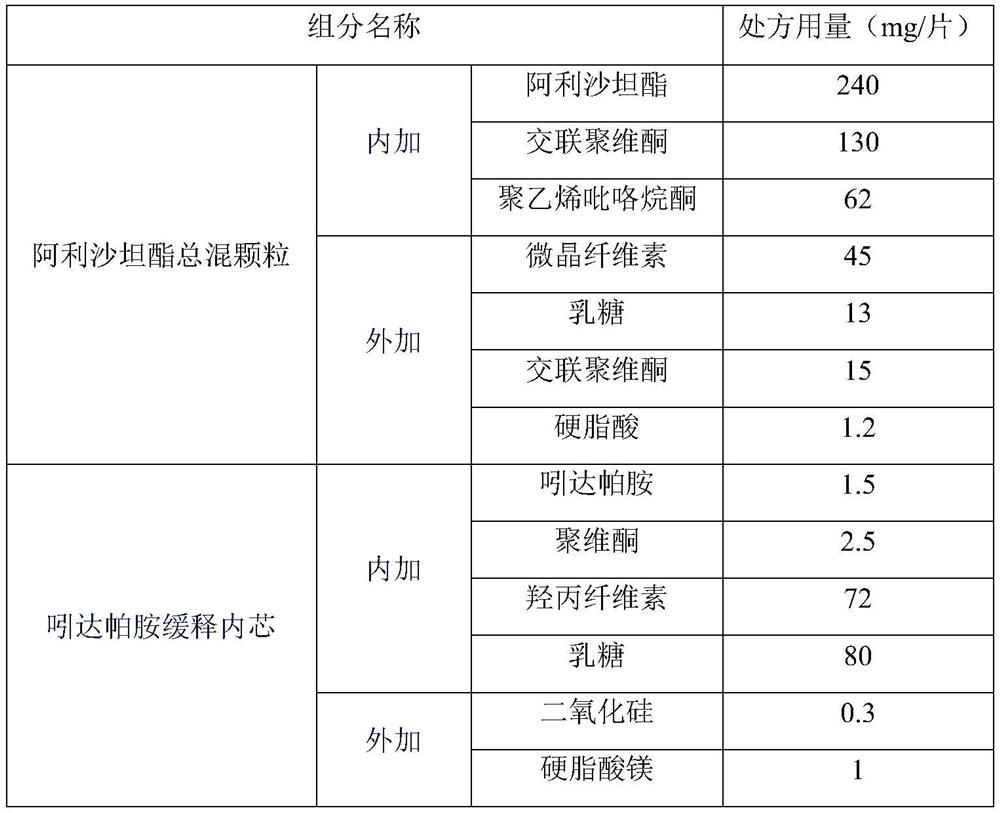
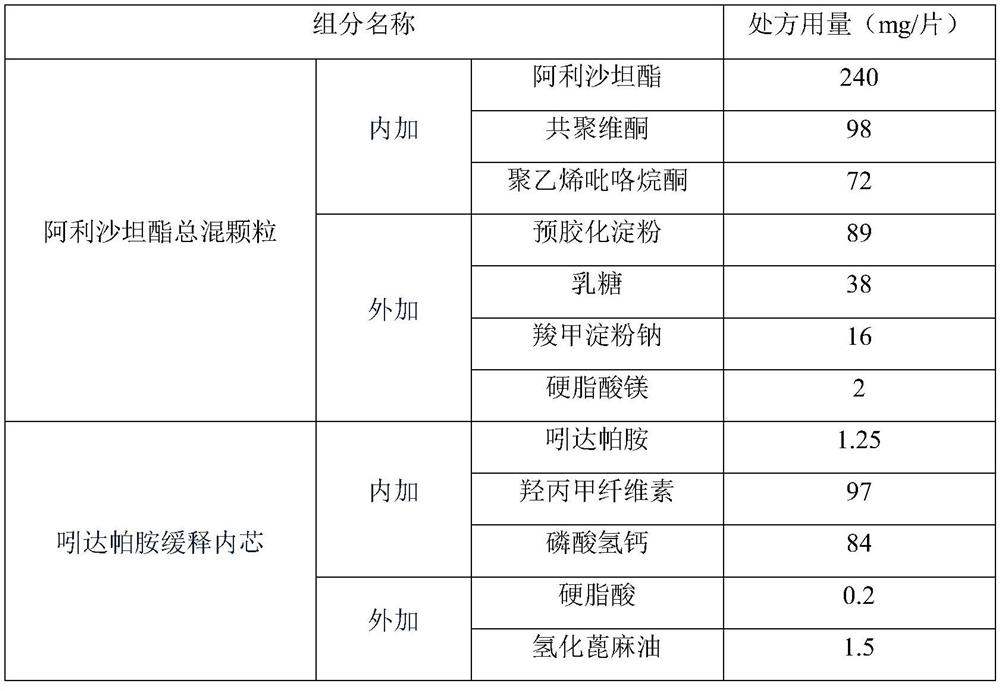
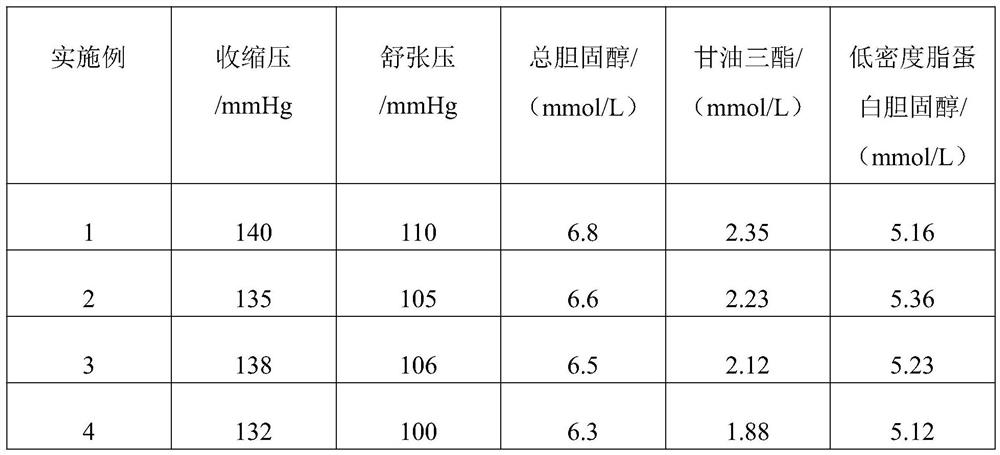
ActiveCN114306263AGuaranteed disintegrationGuaranteed DissolutionOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical drugAllisartan isoproxil
The invention belongs to the technical field of pharmaceutical preparations, and relates to a compound antihypertensive pharmaceutical composition and a preparation method thereof, in particular to a pharmaceutical composition of allisartan medoxomil and / or salt thereof and indapamide and a preparation method thereof.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Hypotensive drug and preparation method thereof
PendingCN114191477APromote blood circulationPromote circulationHydroxy compound active ingredientsMetabolism disorderSide effectAntihypertensive medication
The invention provides an antihypertensive drug and a preparation method thereof, and relates to the technical field of traditional Chinese medicines. The antihypertensive drug comprises the following components in parts by weight: 10-30 parts of uncaria, 15-30 parts of selfheal, 15-25 parts of ligusticum wallichii, 10-25 parts of saponin, 12-24 parts of folium mori and 18-28 parts of radix puerariae. The preparation method of the antihypertensive medicine comprises the following steps: grinding the raw materials into powder to obtain medicine powder, adding water into the medicine powder, and decocting to obtain liquid medicine. The antihypertensive drug can improve and prevent hypertension, and is safe in action mode and free of side effects. The preparation method of the antihypertensive drug is simple and rapid, convenient to use and suitable for batch production.
Owner:张苗苗
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com