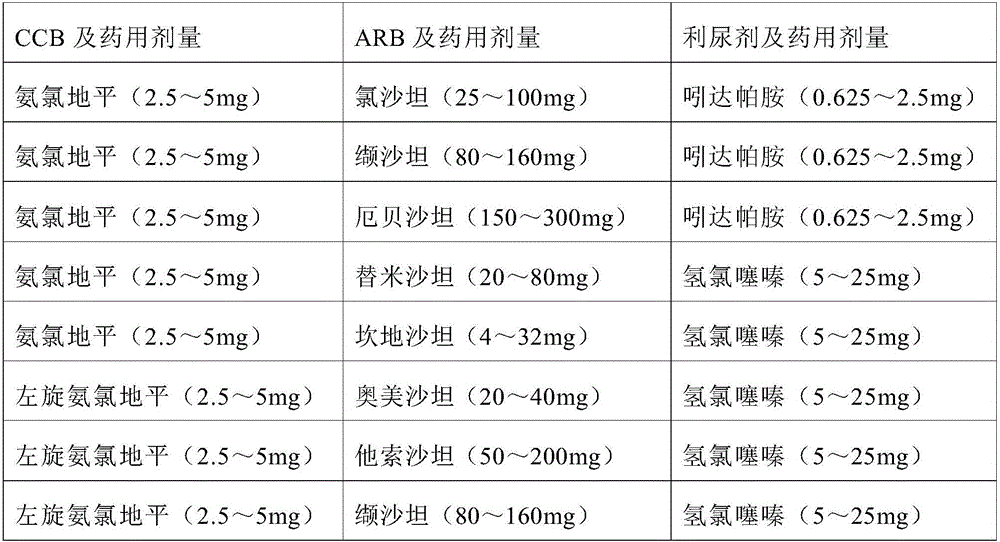
Multi-cascade antihypertensive drug composition containing folic acid
A composition and drug technology, applied in the field of pharmacy, can solve problems such as hypokalemia, short half-life of CCB, inconvenience for patients, etc., and achieve the effects of preventing stroke, enhancing protection, and delaying stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
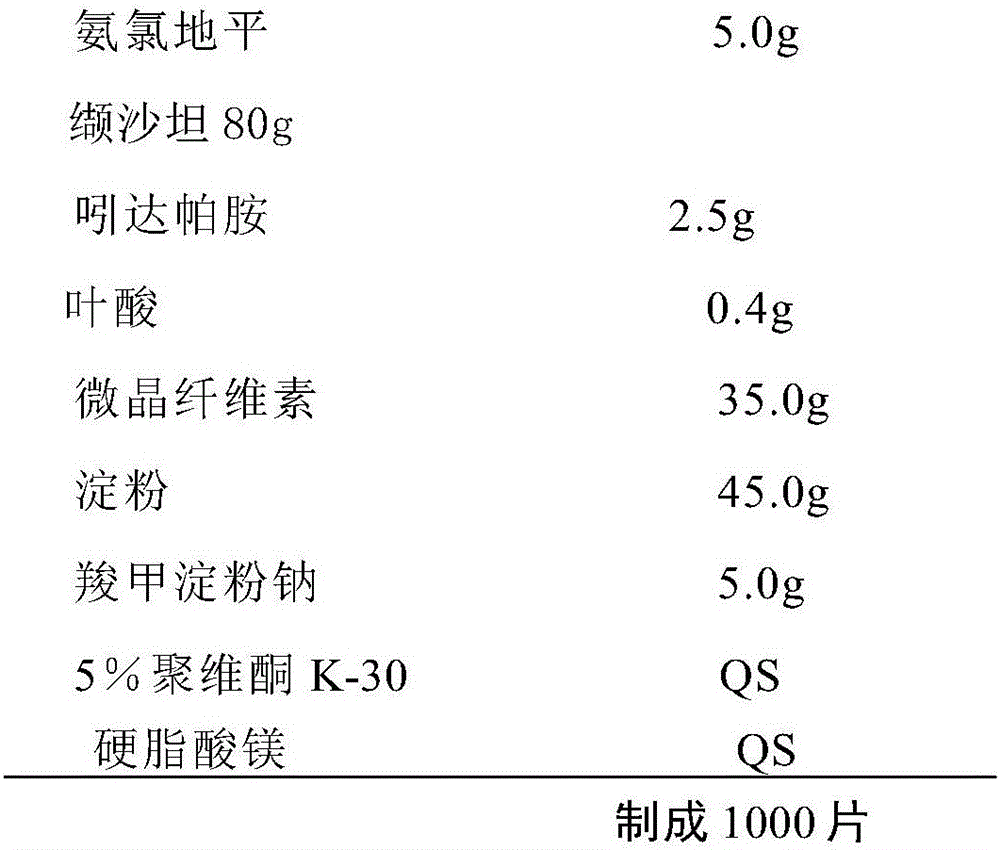
[0053] Example 1: Preparation of Compound Amlodipine 5mg / Valsartan 80mg / Indapamide 2.5mg / Folic Acid 0.4mg
[0054] prescription:
[0055]
[0056] Preparation Process:
[0057] (1) Take the prescribed amount of amlodipine, valsartan, indapamide, and folic acid, pass through a 100-mesh sieve, and mix them uniformly by the method of equal increments;
[0058] (2) Other auxiliary materials are passed through a 100-mesh sieve and dried at 75°C for 2 hours;
[0059] (3) Mix the microcrystalline cellulose, starch and carboxymethyl starch sodium according to the prescription amount, and then mix them with the mixed raw material drugs in equal increments;
[0060] (4) Make soft materials with an appropriate amount of 5% povidone K-30, granulate with a 24-mesh sieve, granulate with a 20-mesh sieve, and dry at 40-45°C;
[0061] (5) Add an appropriate amount of magnesium stearate to the dry granules, mix them evenly, and compress them into tablets after content determination.
Embodiment 2
[0062] Example 2: Preparation of Compound Amlodipine 5mg / Irbesartan 150mg / Indapamide 2.5mg / Folic Acid 0.4mg
[0063] prescription:
[0064]
[0065] Preparation Process:
[0066] (1) Take the prescribed amount of amlodipine, irbesartan, indapamide, and folic acid, pass through a 100-mesh sieve, and mix them uniformly by the method of equal increments;
[0067] (2) Other auxiliary materials are passed through a 100-mesh sieve and dried at 75°C for 2 hours;
[0068] (3) Mix the microcrystalline cellulose, starch and carboxymethyl starch sodium according to the prescription amount, and then mix them with the mixed raw material drugs in equal increments;
[0069] (4) Make soft materials with an appropriate amount of 5% povidone K-30, granulate with a 24-mesh sieve, granulate with a 20-mesh sieve, and dry at 40-45°C;
[0070] (5) Add an appropriate amount of magnesium stearate to the dry granules, mix them evenly, and compress them into tablets after content determination.
Embodiment 3
[0071] Example 3: Preparation of Compound Nicardipine 65mg / Telmisartan 20mg / Hydrochlorothiazide 10mg / Folic Acid 0.8mg
[0072] prescription:
[0073]
[0074] Preparation Process:
[0075] (1) Get the nicardipine, telmisartan, hydrochlorothiazide, folic acid of prescription quantity, after crossing 100 mesh sieves, mix uniformly by the method of equal increments for subsequent use;
[0076] (2) Other auxiliary materials are passed through a 100-mesh sieve and dried at 75°C for 2 hours;
[0077] (3) mix the microcrystalline cellulose, starch, carboxymethyl starch sodium according to the prescription amount and then mix with the mixed raw material drug in equal increments;
[0078] (4) Make soft material with an appropriate amount of 5% povidone K-30, granulate with 24 mesh sieve, granulate with 20 mesh sieve, and dry at 40-45°C;
[0079] (5) Add an appropriate amount of magnesium stearate to the dry granules, mix them evenly, and compress them into tablets after content d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com