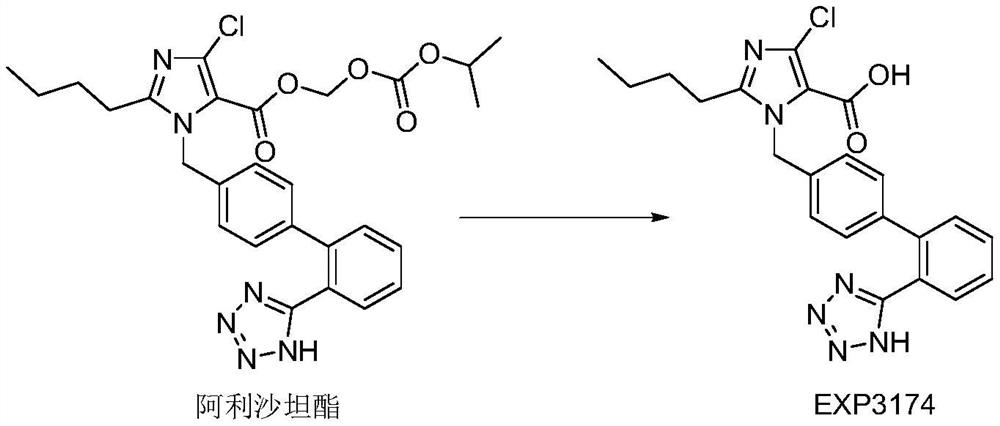
Compound antihypertensive pharmaceutical composition and preparation method thereof
A technology of composition and medicine, which is applied in the field of compound antihypertensive pharmaceutical composition and its preparation, the pharmaceutical composition of alisartan medoxomil and/or its salt and indapamide and its preparation field, which can solve the problem that the equivalence cannot be achieved and other issues, to achieve the effect of reducing treatment costs, ensuring safety and effectiveness, and maintaining blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
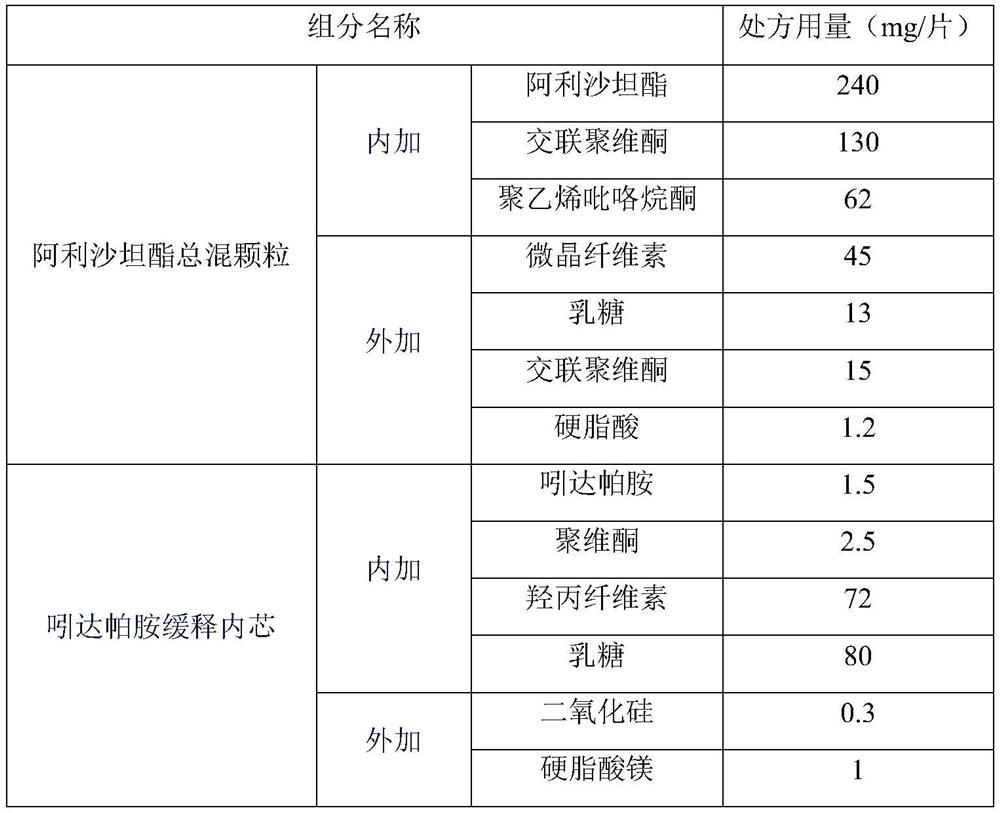
[0036] Calculate and weigh each component of the corresponding prescription amount, and then prepare the alisartan medoxomil indapamide preparation according to the following preparation process, and the specific prescription composition is as follows.
[0037]
[0038] Concrete preparation process is as follows:
[0039](1), Alisartan medoxomil and polyvinylpyrrolidone weighing prescription quantity are dissolved in appropriate methylene chloride, dehydrated alcohol, are made into medicine solution, and the crospovidone of prescription quantity is placed in fluidized bed, The drug solution is sprayed into the fluidized bed for spray granulation and drying, and then the prescribed amount of lactose, microcrystalline cellulose, crospovidone and stearic acid are added for mixing to obtain the total mixed granules of alisartan medoxomil.
[0040] (2), add the prescription amount of povidone to absolute ethanol and purified water, stir until completely dissolved, add the prescr...
Embodiment 2
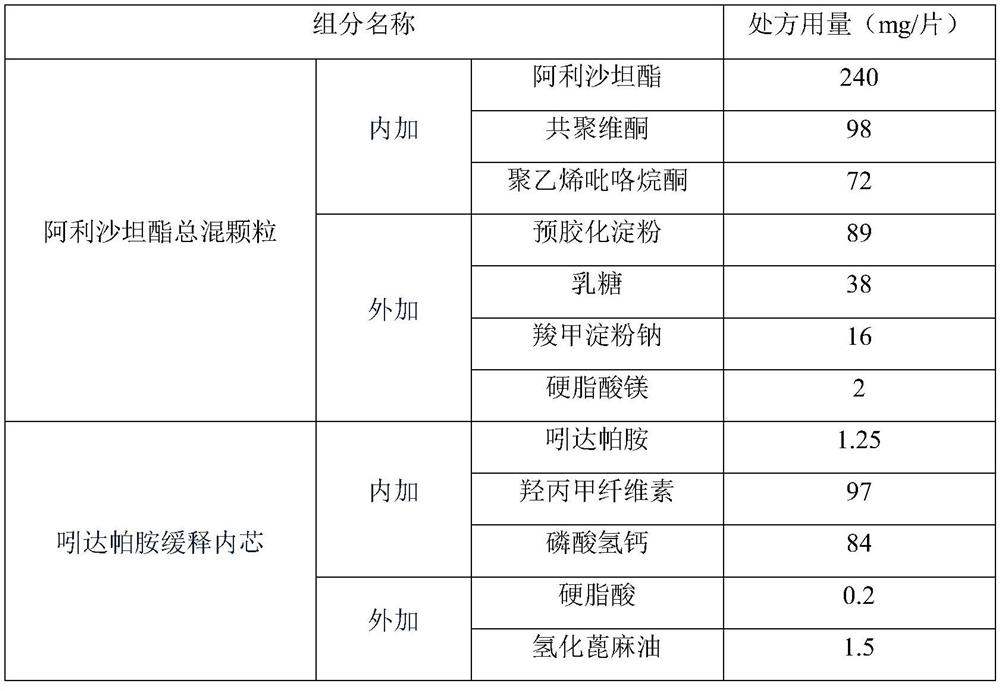
[0044] prescription
[0045]
[0046] preparation:
[0047] (1), dissolving alisartan medoxomil and polyvinylpyrrolidone in an appropriate amount of dichloromethane and absolute ethanol to make a drug solution, place the copovidone in the prescribed amount in a fluidized bed, and spray it into The drug solution is subjected to fluidized-bed spray granulation and drying, and then the prescription amount of lactose, pregelatinized starch, carboxymethyl starch sodium and magnesium stearate are added for mixing to obtain the allisartan medoxomil granules.
[0048] (2) After mixing the prescribed amount of indapamide, hypromellose and calcium hydrogen phosphate, add them into a dry granulator to compress large tablets, and then crush the granules to obtain granules, and then mix them with the added stearin acid and hydrogenated castor oil are mixed together, and the tablets are compressed and coated to obtain indapamide sustained-release tablet cores.
[0049] (3), the alisart...
Embodiment 3
[0052] prescription
[0053]
[0054] Preparation: (1), dissolving alisartan medoxomil and copovidone in an appropriate amount of dichloromethane and absolute ethanol to make a drug solution, and placing the prescribed amount of crospovidone in a fluidized bed , spray the drug solution, carry out fluidized bed spray granulation and drying, and then add lactose monohydrate, mannitol, crospovidone and magnesium stearate in the prescribed amount for mixing to obtain allisartan medoxomil granules.
[0055] (2), mix the prescription amount of indapamide, hypromellose and calcium hydrogen phosphate with additional silicon dioxide and stearic acid, and carry out tablet coating to obtain indapamide sustained-release tablets core.
[0056] (3), the alisartan medoxomil blended granules weighing the prescription amount are the outer layer, and the indapamide sustained-release tablet is the tablet core to compress and pack the chip;
[0057] (4) Chip coating: Add the film coating pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com