An osmium-containing conjugated polymer and its preparation method and application
A conjugated polymer and conjugated technology, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc., can solve problems that hinder performance application research, polymer synthesis difficulties, etc., and achieve 100% atomic conversion efficiency , Molecular weight distribution extremely, the effect of mild condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
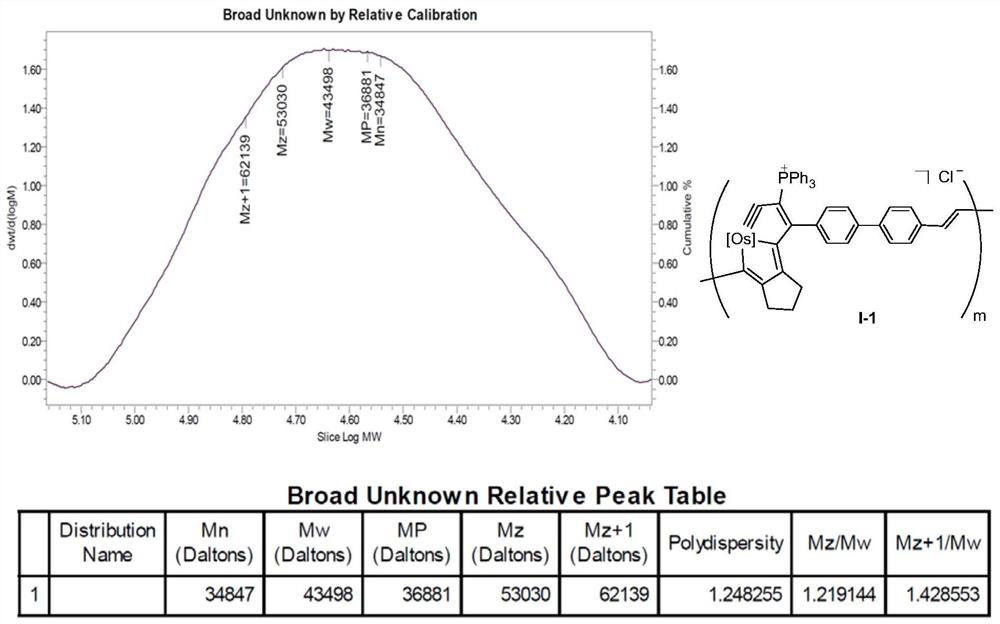
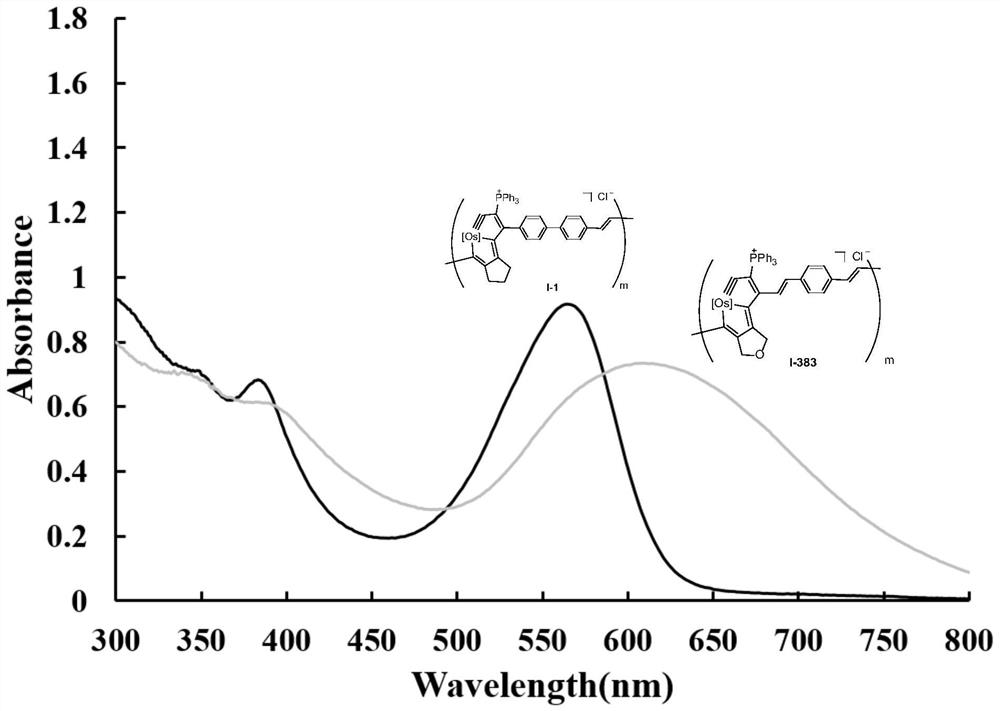
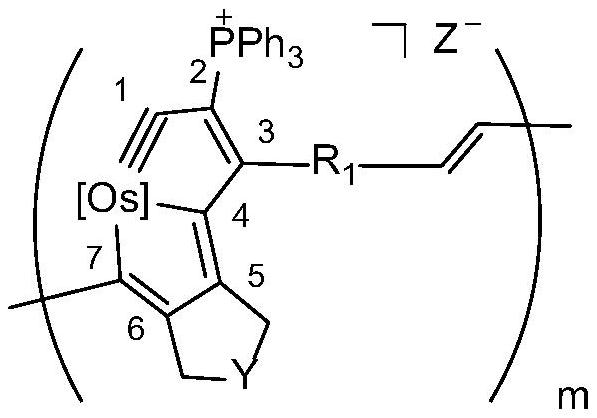
[0090]
[0091] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (Phenylacetylene, purchased from Bailingwei Technology Co., Ltd., the trade name is 445058), and the DCM used is dichloromethane redistilled solvent.
[0092] Preparation of the above-mentioned osmium-containing conjugated polymer: under nitrogen atmosphere, under magnetic stirring, dissolve the monomeric metal heterocyclic ospentyne compound M1 (0.1 mmol) in 5 mL of dichloromethane, and quickly add 2 mol / 0.5 mL of ether hydrochloride solvent in 0.5 mL. After reacting at room temperature for 24 hours, add phenylacetylene to terminate the reaction. After reacting at room temperature for 2 hours, add a large amount of ether, a purple-red solid compound precipitates, filter, and wash the solid product repeatedly with ether. After drying, 90 mg of the purple-red...
Embodiment 2
[0098]
[0099] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (Phenylacetylene, purchased from Bailingwei Technology Co., Ltd., the trade name is 445058), and the DCM used is dichloromethane redistilled solvent.
[0100] Preparation of the above-mentioned osmium-containing conjugated polymer: under nitrogen atmosphere, under magnetic stirring, dissolve the monomeric metal heterocyclic os-pentyne compound M2 (0.1 mmol) in 5 mL of dichloromethane, and quickly add 2 mol / 0.5 mL of ether hydrochloride solvent in 0.5 mL. After reacting at room temperature for 24 hours, add phenylacetylene to terminate the reaction. After reacting for another 2 hours at room temperature, add a large amount of ether, and a purple solid compound precipitates. Filter and wash the solid product repeatedly with ether. After drying, 92 mg of the pu...
Embodiment 3
[0104]
[0105] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (Phenylacetylene, purchased from Bailingwei Technology Co., Ltd., the trade name is 445058), and the DCM used is dichloromethane redistilled solvent.
[0106] Preparation of the above-mentioned osmium-containing conjugated polymer: under nitrogen atmosphere, under magnetic stirring, dissolve the monomeric metal heterocyclic os-pentyne compound M3 (0.1 mmol) in 5 mL of dichloromethane, and quickly add 2 mol / 0.5 mL of ether hydrochloride solvent in 0.5 mL, after reacting at room temperature for 24 hours, add phenylacetylene to terminate the reaction, after reacting at room temperature for 2 hours, add a large amount of ether, a blue solid compound precipitates, filter, and wash the solid product repeatedly with ether, After drying, 95 mg of the osmium-containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com