Culture system and method for inducing human pluripotent stem cells into neural mesoderm progenitor cells in vitro and maintaining self-renewal and application
A technology of human pluripotent stem cells and culture system, applied in the field of long-term culture of neuromesoderm progenitor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
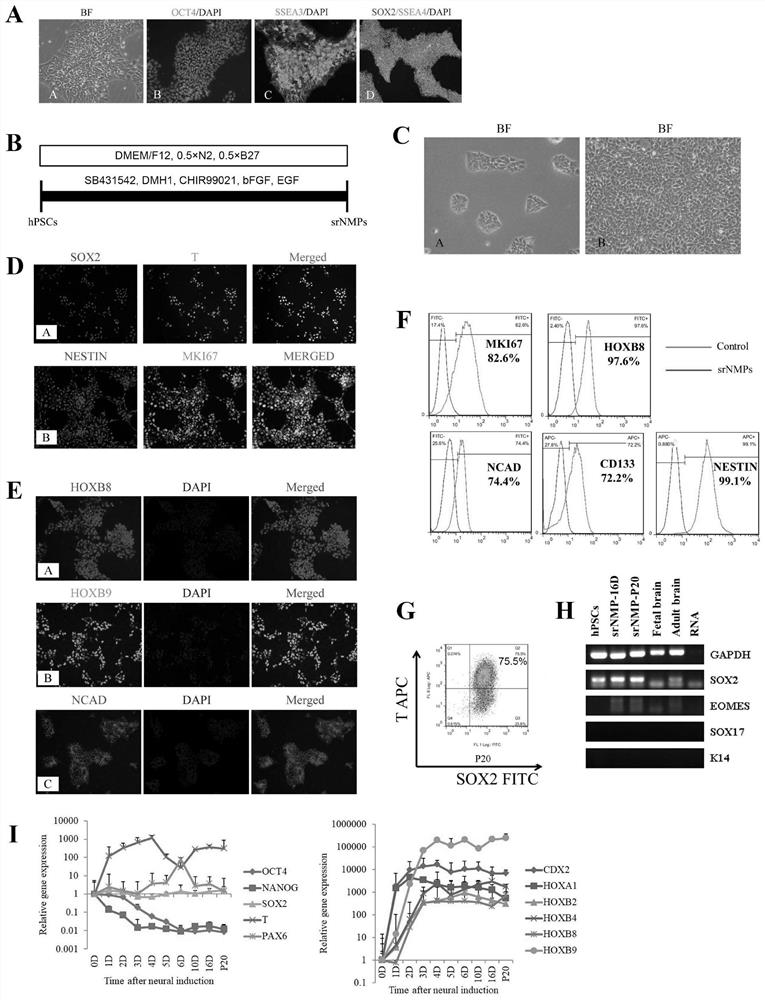
[0035] Example 1: Induction of srNMPs and identification of molecular phenotypes
[0036] 1. Recovery, culture and identification of hPSCs
[0037] The surface of the culture dish of the cells was pre-coated with DMEM / F12 medium containing 1% Matrigel (Corning, Cat#356231), and stored overnight at 4°C. The frozen hPSCs lines in our laboratory include H1 (passage 70-85) and HUES9 (passage 20-35). After taking it out from the -80°C refrigerator, shake it quickly in a 37°C water bath to dissolve until a little ice remains. Centrifuge the cells quickly in a low-temperature horizontal centrifuge, centrifuge at 1000rpm for 5 minutes, discard the frozen storage solution, resuspend with hPSCs medium, inoculate in matrigel-coated culture plates, and change the medium every day. After the cells were digested with TrypLE digestion enzyme (Gibco, Cat# 12605-010), they were subcultured at a ratio of 1:10, and 5 μM Blebbistatin (Selleck, S7099) was added to prevent cell apoptosis caused b...
Embodiment 2
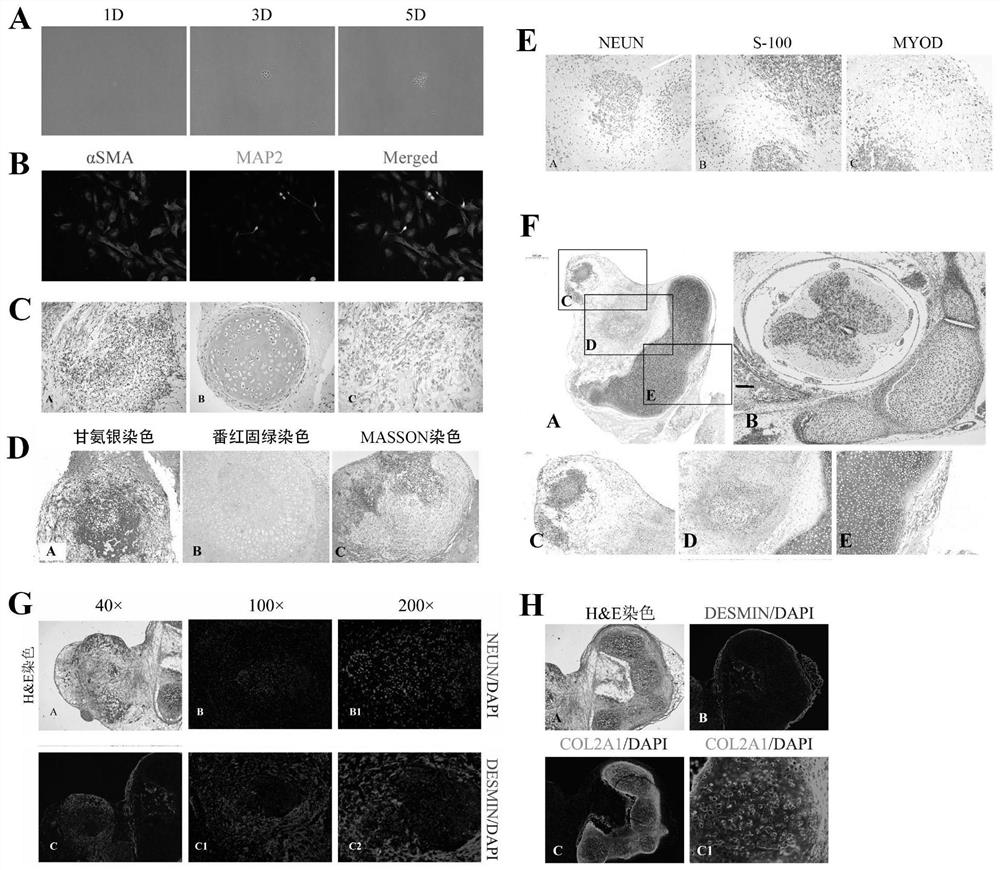
[0050] Example 2: srNMPs have bidirectional differentiation potential of nerve and mesoderm
[0051] 1. Single cell differentiation experiment of srNMPs:
[0052] After srNMPs were digested into single cells, they were inoculated into Matrigel-coated six-well plates at a very low density (about 50 cells per well). After five days of growth under srNMPs culture conditions, most of the single cells formed colonies ( figure 2 A), and then changed to N2B27 basal medium conditions to allow spontaneous differentiation, and continued to culture for two to three weeks, and detected srNMPs clones derived from single cells by immunofluorescence staining, and expressed neural molecular marker MAP2 and mesoderm molecular marker αSMA ( figure 2 B), which shows that the clone formed by a single srNMPs cell can be differentiated to produce neurons and mesoderm cells, and it is proved from the single cell level that srNMPs have the bidirectional differentiation potential of nerves and mesod...
Embodiment 3
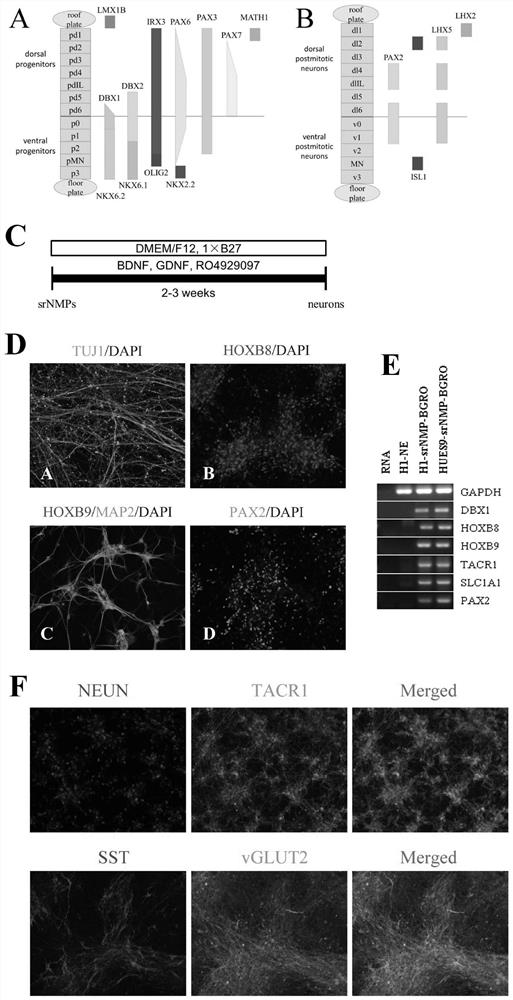
[0057] Example 3: Neural Directed Differentiation of srNMPs
[0058] 1. Spontaneous differentiation of srNMPs towards nerves
[0059]Neural differentiation of srNMPs was continuously induced for three weeks in a neural differentiation medium that did not contain any morphogens. The culture conditions were based on B27 medium, adding 0.2 μM γ-secretase inhibitor RO4929097 (EMD Chemicals), 10 ng / mL BDNF ( PeproTech, 450-02), 10ng / mL GDNF (PeproTech, 450-10), this neuron differentiation condition is recorded as BGRO ( image 3 C).
[0060] Neurons differentiated by srNMPs under BGRO conditions can express broad-spectrum neural molecular markers TUJ1, MAP2 and HOX family HOXB8, HOXB9 ( image 3 D. image 3 E), which shows that the induced neurons still maintain their localization at the posterior end of the spinal cord. The expression of DBX1 and PAX2, the molecular markers of V0 region, and the expression of glutamate transporter gene SLC1A1 were detected by RT-PCR ( image ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com