A kind of preparation method and application of universal bacterial vaccine
A technology for bacteria and vaccines, applied in the field of molecular biology, can solve problems such as difficult, difficult biofilm assembly, and technical difficulties, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
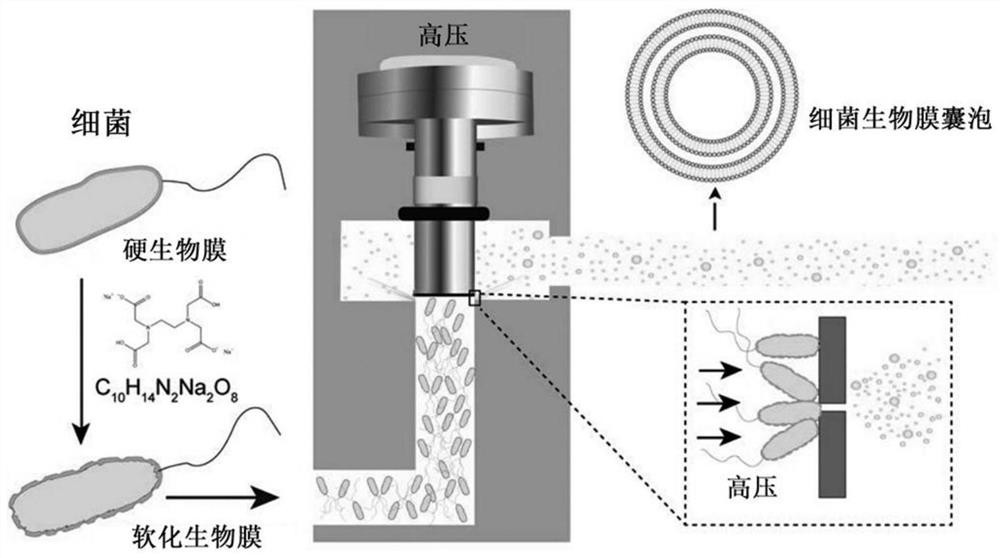
[0060] The present embodiment provides a kind of preparation method of general bacterial vaccine, comprises the following steps:
[0061] Step 1, the softening of bacterial biofilm: cultivate drug-resistant bacteria in the culture medium, when OD600 reaches 1.0, put C 10 h 14 N 2 Na 2 o 8 (EDTA·2Na) was slowly added to the culture medium until the concentration reached 10mM, incubated with shaking at 37°C for 30 minutes, then centrifuged the culture medium at 14,000×g for 20 minutes, removed the supernatant, and used HBSS (without Ca 2+ , Mg 2+ and phenol red, Servicebio) and resuspended in 300ml HBSS to obtain a bacterial suspension.
[0062] The drug-resistant bacteria include carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Escherichia coli, and methicillin-resistant aureus Staphylococci, and vancomycin-resistant Enterococcus faecalis. The above bacteria were provided by the Second Affiliated Hospital of ...
Embodiment 2
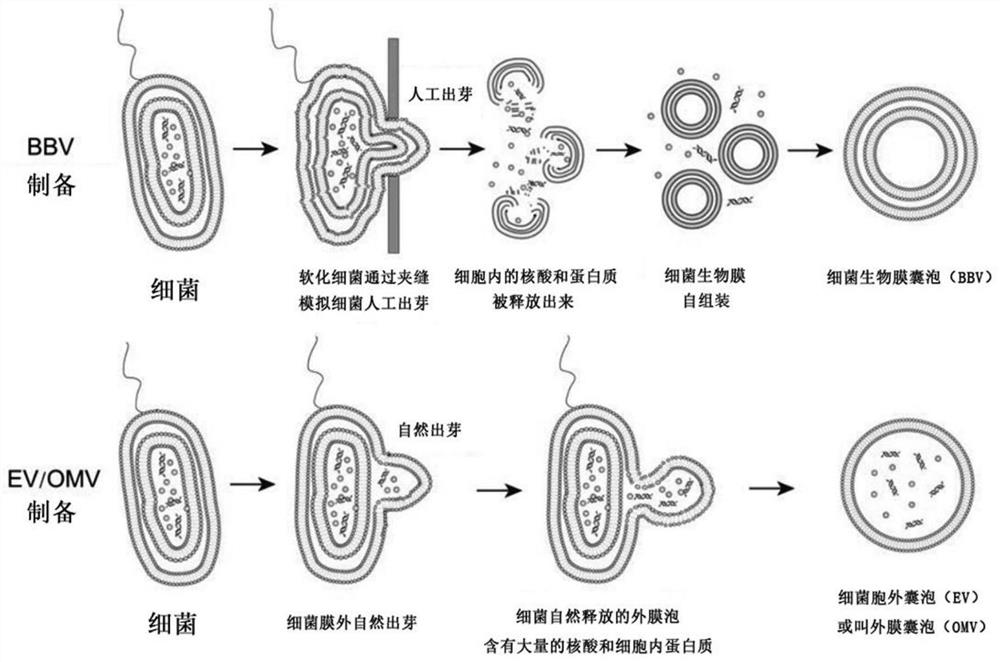
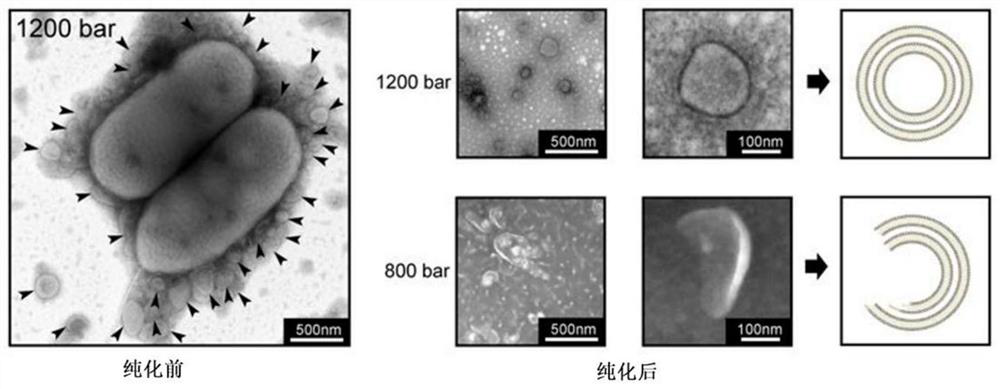
[0072] In order to test the influence of different pressure intensities on the preparation of BBV, the method described in Example 1 was used in this example to prepare the BBV of Klebsiella pneumoniae, which were prepared under pressures of 200 bar, 400 bar, 800 bar, and 1200 bar respectively. The results proved that under the pressure of 1200bar, the bacteria can be driven to form a large number of complete BBVs, while the vesicles can also be formed under the conditions of 200bar, 400bar, and 800bar, but the structure is not complete.
[0073] Such as image 3 , showing representative electron micrographs of BBV driven by 800bar and 1200bar pressures. The left figure shows that after 1200bar pressure drives Klebsiella pneumoniae before purification, a large amount of complete BBV can be seen. The 1200bar and 800bar pressure-driven electron micrographs of purified bacteria in the right figure show that a large number of complete vesicles can be formed under the drive of 120...
Embodiment 3
[0076] Embodiment 3——BBV generation mechanism
[0077] In order to clarify the generation mechanism of BBV, the following experiments were carried out in this embodiment:
[0078] The Kp-BBV samples purified in Example 1, and the Kp whole bacteria, Kp lysate, and Kp-EV described in Comparative Example 1 were analyzed on 10% SDS-PAGE electrophoresis. Such as Figure 4 The SDS-PAGE analysis shown shows that Kp-BBV has the least protein composition.
[0079] The ITRAQ proteomics analysis of the Kp-BBV and the whole bacterial body proteins showed that 291 proteins were increased and 117 proteins were decreased in BBV compared with the whole bacterial body of Kp. Cluster analysis proved that the up-regulated proteins of Kp-BBV compared with Kp whole cells were mostly proteins on the biofilm, and most of the reduced proteins were intracellular proteins; GO enrichment analysis proved that Kp-BBV and Kp whole cells were up-regulated or down-regulated Most of the proteins in Kp-BBV ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com