Liquid chromatography-mass spectrometry detection method for potential genotoxic impurities in irbesartan
An impurity, mass spectrometry technology, applied in instruments, measuring devices, scientific instruments, etc., can solve the problem that the detection sensitivity cannot meet the requirements and has not yet been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
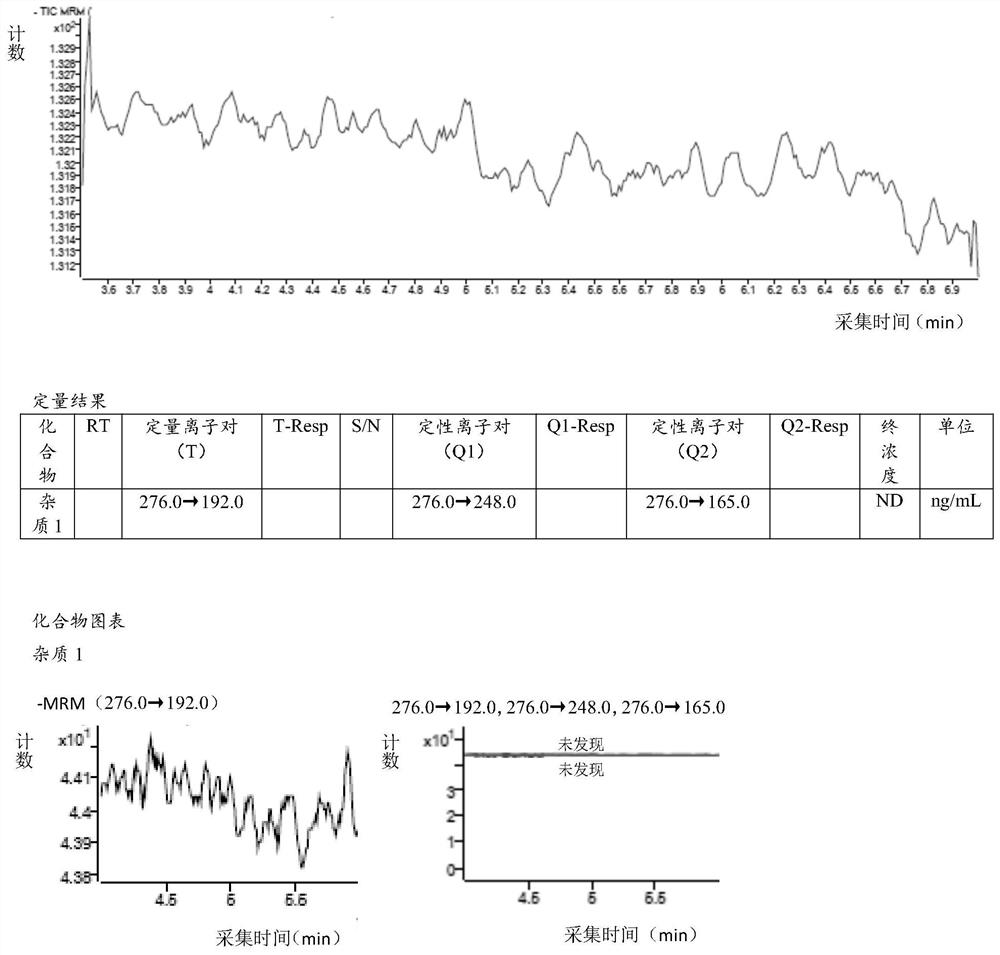
[0094] Embodiment 1 specificity verification
[0095] 1.1 Diluent (acetonitrile-water (80:20)): Measure 200ml of ultrapure water in 800ml of acetonitrile and mix well;
[0096] 1.2 Impurity 1 stock solution:
[0097] Take 30.55mg of impurity 1 working reference substance, put it in a 100ml volumetric flask, dissolve it with diluent and dilute to the mark, and shake well. Accurately measure 5ml of the above solution, place it in a 100ml volumetric flask, dilute to the mark with diluent, and shake well. Then accurately measure 1ml of the above solution, place it in a 100ml volumetric flask, dilute to the mark with diluent, shake well, and use it as the impurity 1 stock solution, the concentration of impurity 1 in the solution is 152.75ng / ml.
[0098] 1.3 Reference substance solution:
[0099] Accurately measure 1ml of the stock solution of impurity 1 prepared in step 1.2 of this example into a 10ml volumetric flask, dilute to the mark with diluent, and shake well to obtain th...
Embodiment 2
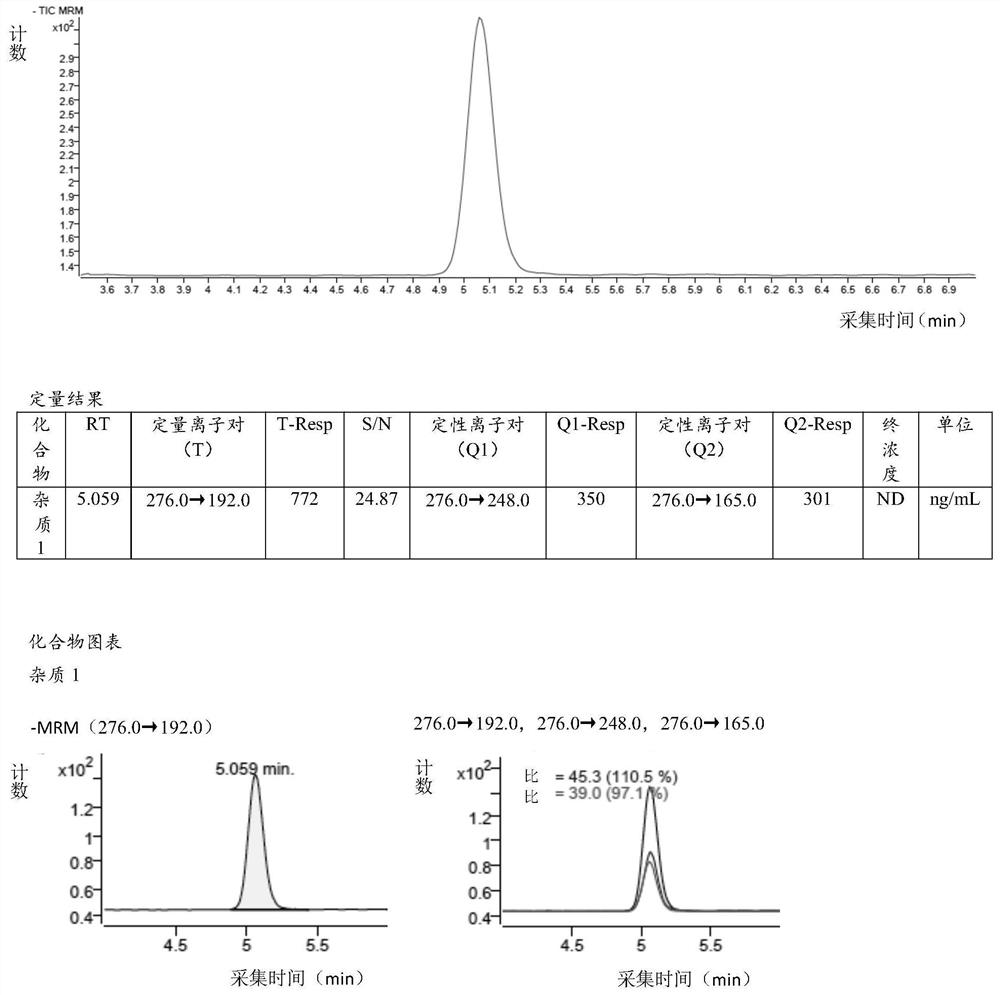
[0115] Example 2 Quantitative limit and detection limit verification
[0116] 2.1 Diluent (acetonitrile-water (80:20)): Measure 200ml of ultrapure water in 800ml of acetonitrile and mix well;
[0117] 2.2 Reference substance solution:
[0118] Precisely measure 1ml of the impurity 1 stock solution prepared in step 1.2 in the specificity verification of Example 1 in a 10ml volumetric flask, dilute to the mark with diluent, shake well, and obtain the reference substance solution. The concentration of impurity 1 in the solution is 15.2750 ng / ml.
[0119] 2.3 Quantitation limit test solution:
[0120] Measure 0.8ml of the reference substance solution prepared in step 2.2 of this embodiment in a 10ml volumetric flask, dilute to the mark with a diluent, and shake up to obtain the limit of quantitation test solution. The concentration of impurity 1 in the solution is 1.2220ng / ml.
[0121] 2.4 Detection limit test solution:
[0122] Measure 3.3ml of the limit of quantitation test ...
Embodiment 3
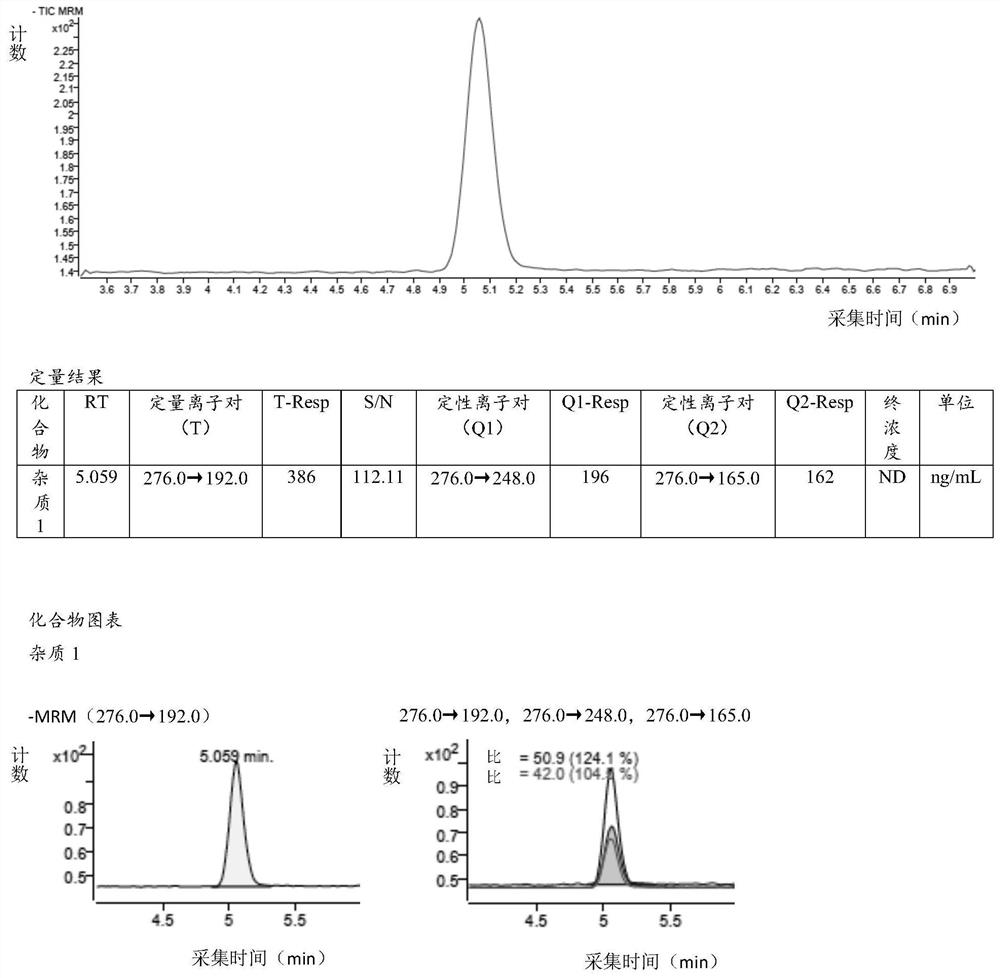
[0137] Example 3 Linearity and Range Verification
[0138] 3.1 Diluent (acetonitrile-water (80:20)): Measure 200ml of ultrapure water in 800ml of acetonitrile and mix well;
[0139] 3.2 Reference substance solution:
[0140] Precisely measure 1ml of the impurity 1 stock solution prepared in step 1.2 in the specificity verification of Example 1 in a 10ml volumetric flask, dilute to the mark with diluent, shake well, and obtain the reference substance solution. The concentration of impurity 1 in the solution is 15.2750 ng / ml.
[0141] 3.3 Sensitivity solution:
[0142] Measure 0.8ml of the reference substance solution prepared in step 3.2 of this example into a 10ml volumetric flask, dilute to the mark with diluent, shake well, and obtain the sensitivity solution. The concentration of impurity 1 in the solution is 1.2220ng / ml.
[0143] 3.4 Linearity test solution
[0144] Linearity test solution 1: Measure 0.8ml of the reference solution prepared in step 3.2 of this example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com