Battery electrolyte containing amine iodide additive and preparation method of battery electrolyte
An electrolyte and additive technology, which is applied in the field of battery electrolytes containing iodide amine additives and their preparation, can solve the problems of increasing the electrolyte preparation process and cost, and achieves improved long-cycle performance, decreased Coulombic efficiency, and increased lithium deposition size. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Purify the organic solvent dioxolane and 1,2-dimethoxyethane with molecular sieves to remove water;
[0051] (2) In an argon atmosphere (H 2 O2 <0.1ppm), mix the purified 5mL dioxolane and 5mL 1,2-dimethoxyethane evenly to obtain the organic solvent of the electrolyte;
[0052] (3) At room temperature, dissolve 1.435 g of lithium bis(trifluoromethylsulfonyl)imide in an organic solvent, stir well until completely dissolved, and the lithium bis(trifluoromethylsulfonyl)imide The molar concentration is 0.5mol / L;
[0053] (4) Add 0.226g lithium nitrate to the solution obtained in step (3), stir until completely dissolved, and the addition ratio of lithium nitrate is 2wt%, to obtain a blank electrolyte;
[0054] (5) Add 0.0113g of methyl ammonium iodide to the blank electrolyte, stir evenly until completely dissolved, the addition ratio of methyl ammonium iodide is 0.1wt%, and obtain a battery electrolyte containing the ammonium iodide additive.
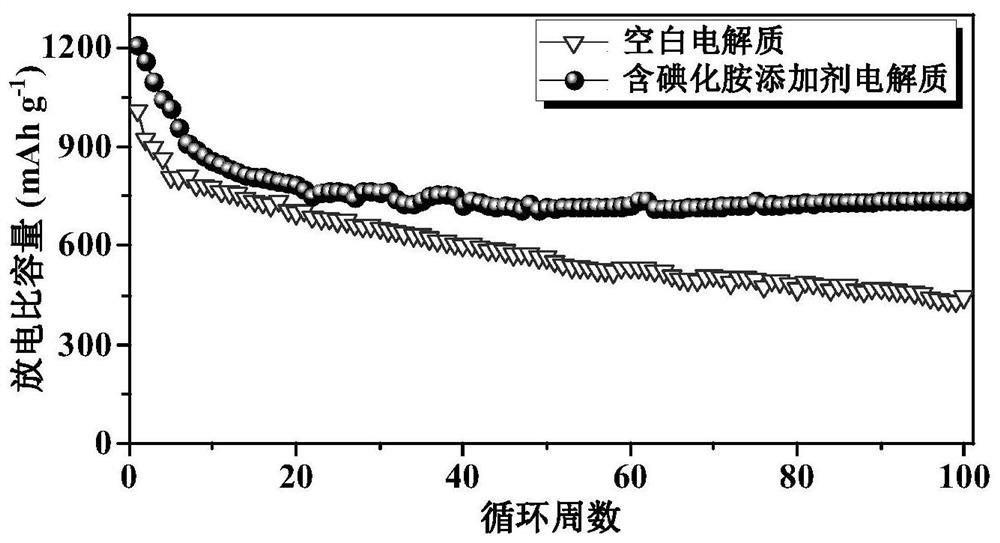
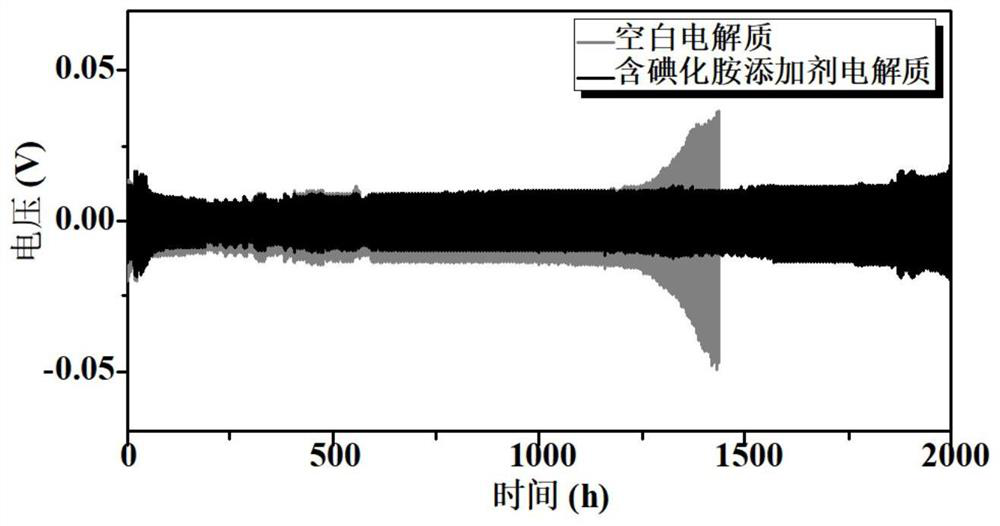
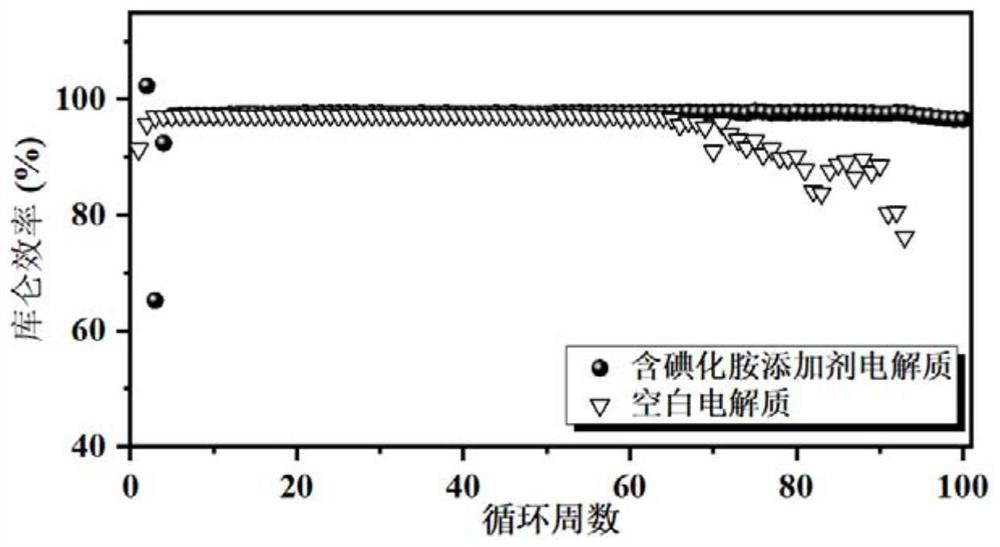
[0055] Two sets of li...
Embodiment 2
[0059] (1) organic solvent dioxolane and triglyme are purified with molecular sieves to remove water;
[0060] (2) In an argon atmosphere (H 2 O2 <0.1ppm), the purified 5mL dioxolane and 5mL triglyme were uniformly mixed to obtain the organic solvent of the electrolyte;
[0061] (3) At room temperature, dissolve 1.435 g of lithium bis(trifluoromethylsulfonyl)imide in an organic solvent, stir well until completely dissolved, and the lithium bis(trifluoromethylsulfonyl)imide The molar concentration is 0.5mol / L;
[0062] (4) Add 0.238g lithium nitrate to the solution obtained in step (3), stir until completely dissolved, and the addition ratio of lithium nitrate is 2wt%, to obtain a blank electrolyte;
[0063] (5) Add 0.0119g of tetramethylammonium iodide to the blank electrolyte, stir until it is completely dissolved, and the addition ratio of the tetramethylammonium iodide additive is 0.1wt%, to obtain a battery electrolyte containing the ammonium iodide additive.
[0064] T...
Embodiment 3
[0068] (1) organic solvent dioxolane and triglyme are purified with molecular sieves to remove water;
[0069] (2) In an argon atmosphere (H 2 O2 <0.1ppm), the purified 5mL dioxolane and 5mL triglyme were uniformly mixed to obtain the organic solvent of the electrolyte;
[0070] (3) At room temperature, dissolve 2.87g of lithium bis(trifluoromethylsulfonyl)imide in an organic solvent, stir well until completely dissolved, the lithium bis(trifluoromethylsulfonyl)imide The molar concentration is 1mol / L;
[0071] (4) Add 0.1785g lithium nitrate to the solution obtained in step (3), stir until completely dissolved, and the addition ratio of lithium nitrate is 1.5wt%, to obtain a blank electrolyte;
[0072] (5) Add 0.0179g of tetramethylammonium iodide to the blank electrolyte, stir until completely dissolved, and the addition ratio of tetramethylammonium iodide additive is 0.15wt%, and obtain the battery electrolyte containing the ammonium iodide additive.
[0073] Two sets of li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com