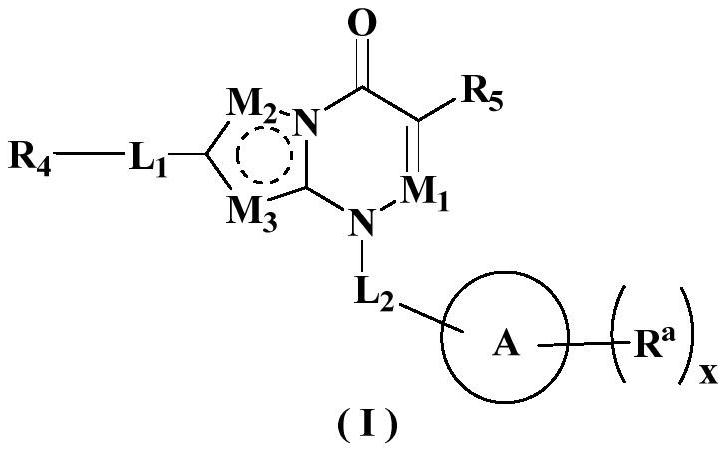
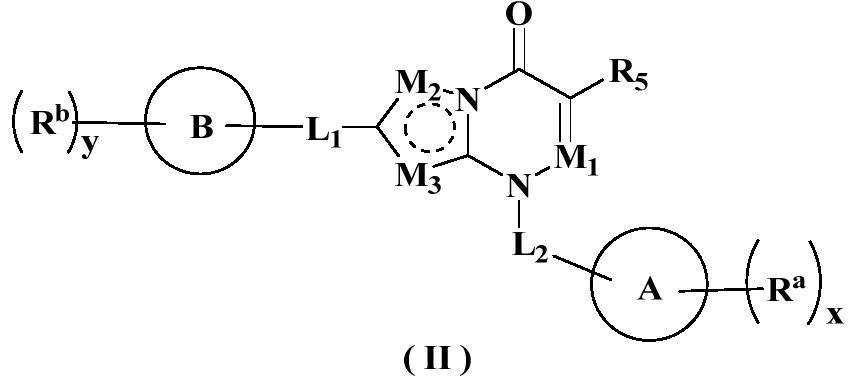
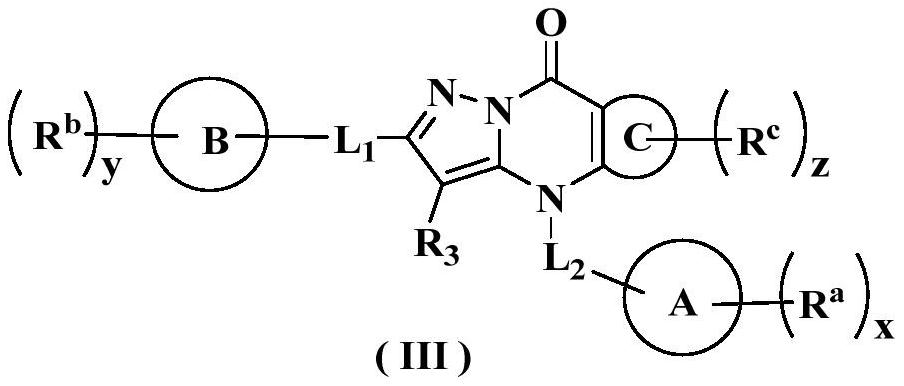
Nitrogen-containing ring derivative inhibitor as well as preparation method and application thereof
A compound and cycloalkyl technology, which is applied in the field of nitrogen-containing ring derivative inhibitors and their preparation, can solve problems such as loss of taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] 4-(4-Chlorobenzyl)-7-oxo-2-(tetrahydro-2H-pyran-4-yl)-N-(2,2,2-trifluoromethyl)-4,7- Dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide
[0237]
[0238] The first step: the preparation of 3-oxo-3-(tetrahydro-2H-pyran-4-yl)propionitrile
[0239]
[0240] A solution of LiHMDS in tetrahydrofuran (26.3 mL, 26.3 mmol) was cooled to -78°C and acetonitrile (1.43 mL, 27.5 mmol) was slowly added dropwise. After the mixture was stirred at -78°C for 1 hour, a solution of methyl tetrahydro-2H-pyran-4-carboxylate (3.6 g, 25.0 mmol) in tetrahydrofuran (12 mL) was slowly added dropwise. After the mixture was stirred at -78°C for 1 hour, the dry ice bath was removed and slowly returned to ambient temperature. The mixture was poured into ice water (250 mL) and extracted three times with methyl tert-butyl ether. The aqueous phase was cooled to 0°C and the pH was adjusted to around 3 with 6M HCl. Extracted with ethyl acetate three times, combined the organic phases, dried and conce...
Embodiment 2
[0263] 4-(4-Chlorobenzyl)-5-fluoro-7-oxo-2-(tetrahydro-2H-pyran-4-yl)-N-(2,2,2-trifluoroethyl)-4 ,7-Dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide
[0264]
[0265] The first step: the preparation of 1,1-diethyl-1-methylmethane tricarboxylic acid
[0266]
[0267] In a 100 mL round bottom flask was added magnesium turnings (1.3 g, 53 mmol) and 8.0 mL of ethanol. 0.5 mL of carbon tetrachloride was added, and the mixture was refluxed under argon for 1 hour. The reaction mixture thickened and turned white. Diethyl malonate (8.0 g, 50 mmol) was added to 10 mL of ethanol and heated for an additional 3 hours to give a dark green solution. The solution was cooled to room temperature and 6.0 mL of methyl chloroformate was added to 10 mL of THF. Heating was continued for over an hour and then stirred overnight at room temperature. The reaction was quenched with saturated ammonium chloride, then extracted with 225 mL of ethyl acetate. The organic layer was dried over anhydro...
Embodiment 3
[0289] 4-(2-((5-Methoxypyridin-2-yl)amino)-2-oxoethyl)-7-oxo-2-(tetrahydro-2H-pyran-4-yl)- N-(2,2,2-Trifluoroethyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide
[0290]
[0291]For the synthesis of Example 3, refer to the method of Example 2, and replace Example 2-5 with Example 2-3 to obtain the target compound (15 mg, 68% yield).
[0292] MS m / z(ESI): 499.9[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com