Method for preparing polycarbonate under catalysis of ionic liquid
A technology of ionic liquid and polycarbonate, which is applied in the field of catalyzed preparation of polycarbonate by ionic liquid, which can solve problems such as toxicity, limited application, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
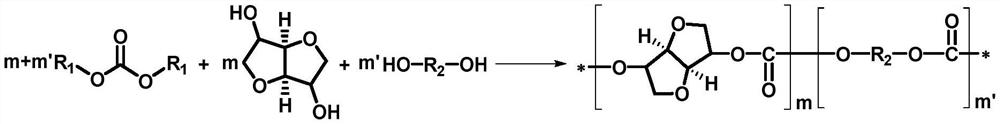
[0063] In this example, isosorbide and dimethyl carbonate are used as raw materials to prepare a polycarbonate under the catalysis of amide ionic liquid, and the reaction formula is as follows:
[0064]
[0065] The preparation method comprises the following steps:
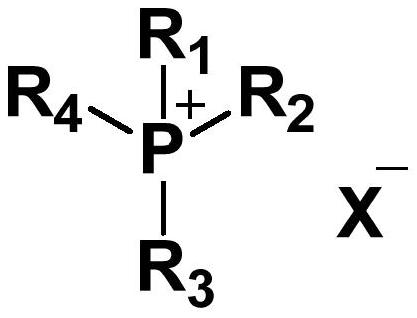
[0066] (1) Transesterification stage: under nitrogen atmosphere and normal pressure, add 7.3g (0.05mol) isosorbide, 22.5g (0.25mol) dimethyl carbonate and tetrabutylphosphorus phthaloyl to the reaction flask Imine (0.0005mol, 0.2025g), react the reaction mixture at 160°C for 2h, then slowly raise the temperature to 180°C, react for 0.5h, volatilize the unreacted dimethyl carbonate and low boiling point product methanol, and obtain the prepolymer ;
[0067] (2) Polycondensation stage: the temperature in step (1) is raised from 180°C to 250°C, the vacuum is slowly reduced to 50Pa, and the reaction is carried out for 1.5h to obtain the product isosorbide polycarbonate;
[0068] Molecular weight test: The GPC tes...
Embodiment 2
[0072] The difference between this example and example 1 is that the catalyst used is tetrabutylphosphoglutarimide (0.0005mol, 0.1855g), and other conditions are exactly the same as those of example 1.
[0073] Molecular weight test: The GPC test result of the product shows that the number average molecular weight of polycarbonate M n 22000g / mol, weight average molecular weight M w It is 37000g / mol.
[0074] Thermal performance test: The DSC test results of the product show that the glass transition temperature T of polycarbonate g It is 163°C.
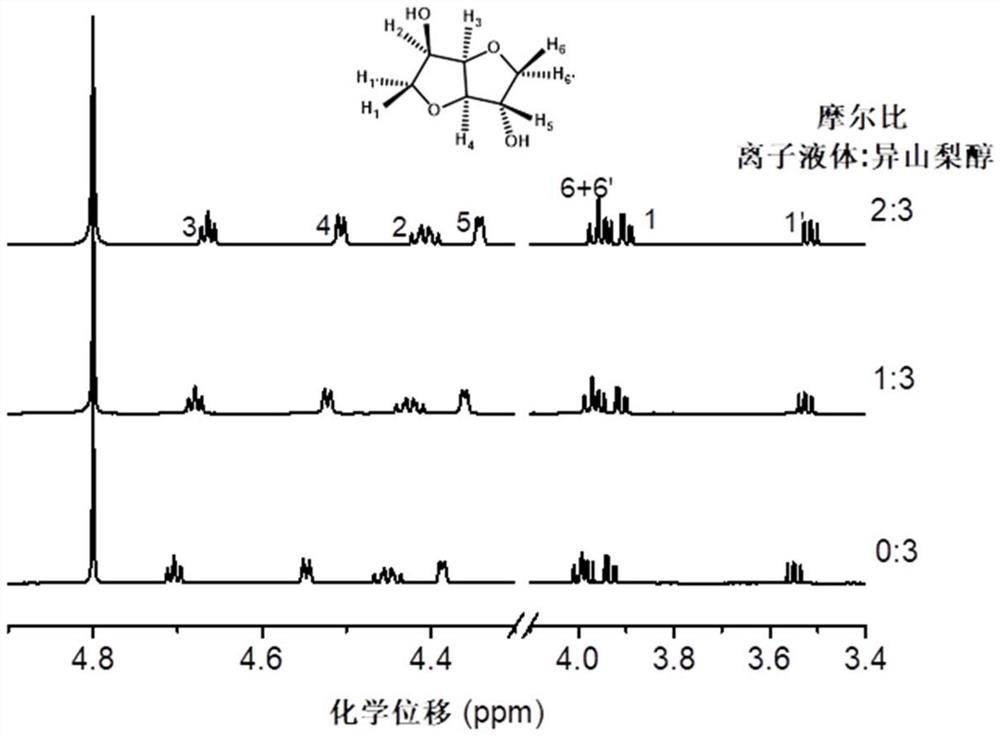
[0075] NMR test: methylation selectivity 0.7%, methyl esterification selectivity 99.3%.
Embodiment 3
[0077] The difference between this example and Example 1 is that the catalyst used is tetrabutylphosphine hexahydrophthalimide (0.0005mol, 0.2055g), and other conditions are exactly the same as those of Example 1.
[0078] Molecular weight test: The GPC test result of the product shows that the number average molecular weight of polycarbonate M n 20000g / mol, weight average molecular weight M w It is 33000g / mol.
[0079] Thermal performance test: The DSC test results of the product show that the glass transition temperature T of polycarbonate g is 160°C.
[0080] NMR test: methylation selectivity 1.3%, methyl esterification selectivity 98.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com