Anti-BCMA antigen binding fragment and application thereof
A technology combining fragments and BCMA-55, applied in the field of biomedicine, can solve the problems of high effective drug clearance rate, failure to solve the recurrence rate of patients, etc., and achieve the effect of improving the killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] This example is used for the construction and panning of the phage nanobody library, and ELISA is used for preliminary screening. Specific steps are as follows:
[0063] (1) Construction of phage nanobody library
[0064] Bactrian camels were immunized with BCMA-Fc expressing the extracellular region, and after the titer was verified by ELISA, 200 mL of peripheral blood was drawn; lymphocytes were sorted, and peripheral blood mononuclear lymphocyte precipitates were obtained, and RNA was extracted; III reverse transcriptase uses RNA as a template to synthesize the first-strand cDNA, and then uses nested PCR to amplify the VHH gene; inserts the VHH gene into the pMECS phage display vector, and after electrotransforming TG1 competent cells, take an appropriate amount of bacterial liquid for library identification , all the remaining cultures were evenly spread on LB / AMPGLU plates, and the lawn was collected after the bacteria grew out, and 1 / 3 of the volume of 50% glyce...
Embodiment 2
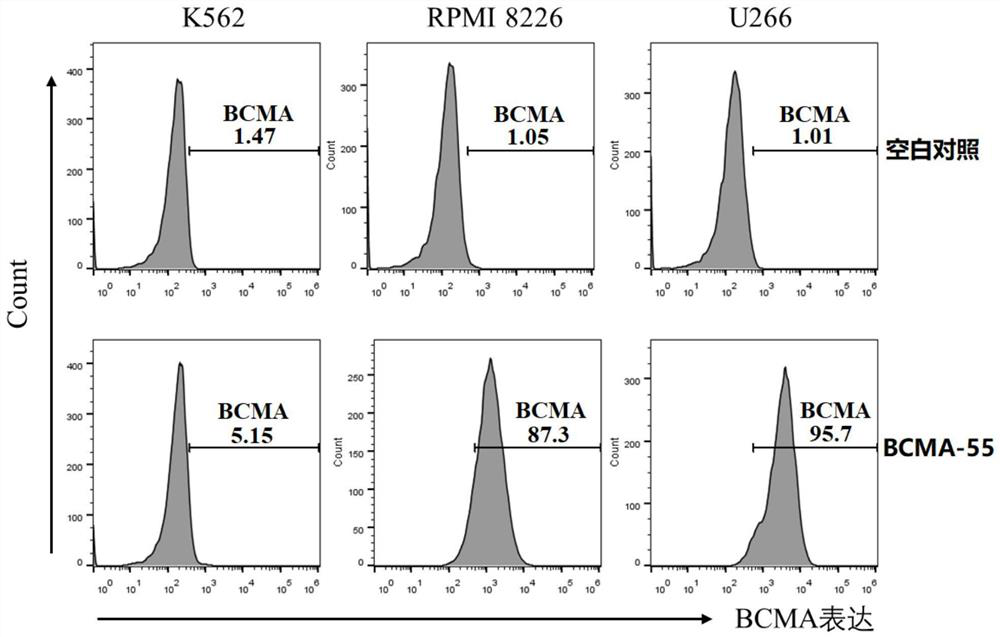
[0078] In this example, the candidate clones were screened using the flow cytometry fluorescence sorting technique (Fluorescence activated Cell Sorting, FACS).
[0079] Carry out cell culture according to the standard cell culture protocol, use trypsin to digest cells to prepare BCMA-positive and negative cell suspensions; 6 cell / mL; Add 2×10 to each well in a V-bottom 96-well plate 5 After centrifuging at 300g for 5min, remove the supernatant, add the crude extract of VHH antibody to resuspend the cells, and incubate at 4°C for 1h;
[0080]After centrifuging at 300g for 5 minutes, remove the supernatant, resuspend the cells in Flow Buffer, dilute the APCanti-his antibody to 2 μg / mL with Flow Buffer, resuspend the cells in 100 μL per well, and incubate at 4 °C for 1 hour; wash the cells with Flow Buffer 3 times, then use 200 μL Flow Buffer Cells were resuspended and analyzed by flow cytometry.
Embodiment 3
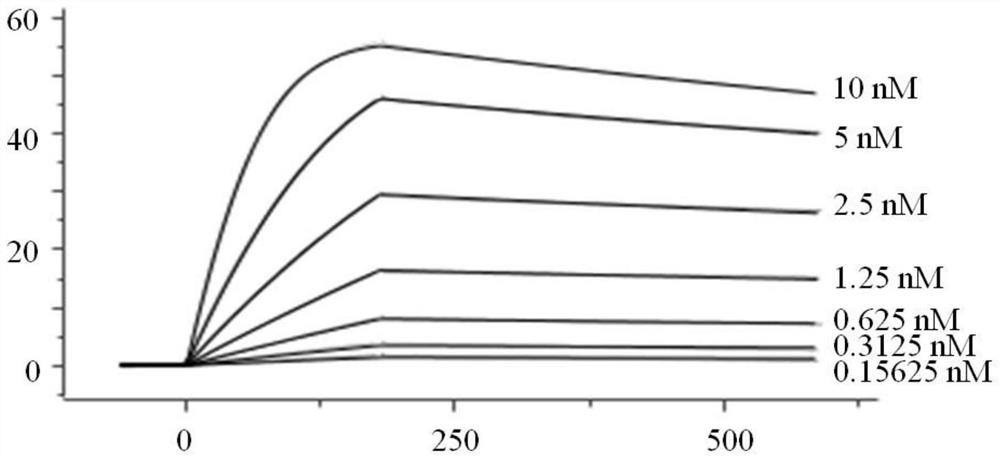
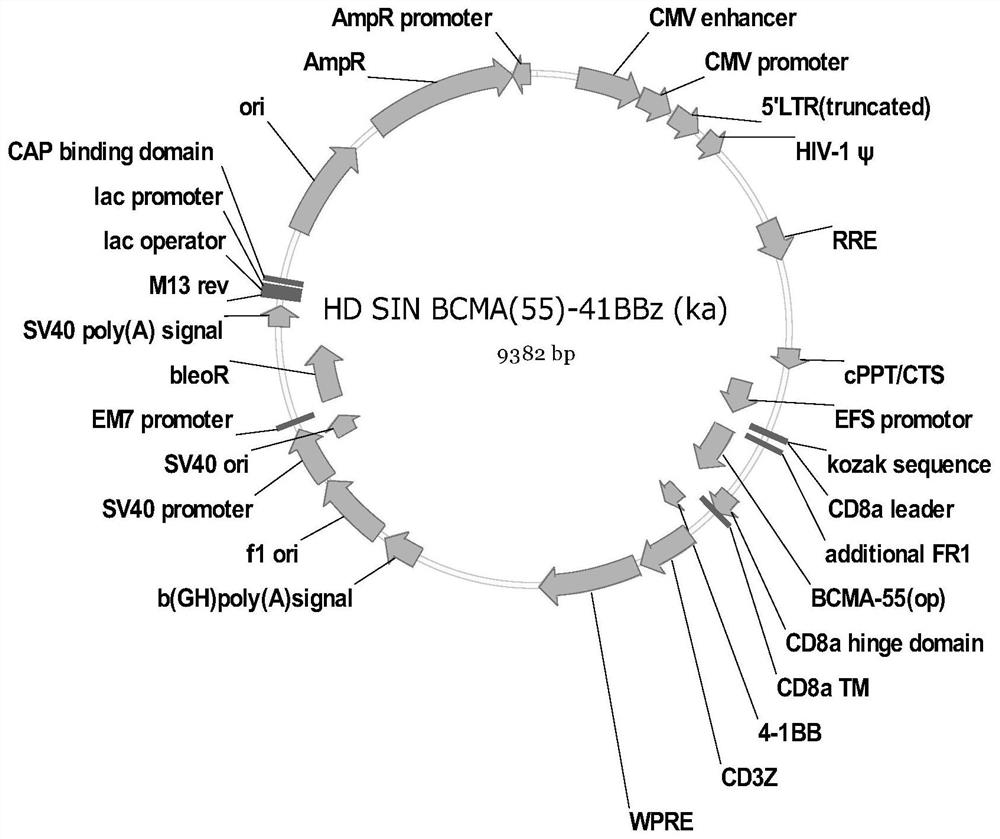
[0082] In this example, the VHH-mIgG2a Fc nanobody was expressed and purified, and the antibody affinity was measured. In order to further identify the screened antibodies, the antibodies need to be expressed by mammalian cells. Therefore, a plasmid vector expressing VHH with a mouse Fc tag was firstly constructed, which was denoted as C-4pCP.Stuffer-mCg2a-FC, and the specific steps were as follows:
[0083] 1. Using PCR to amplify BCMA VHH B55, the primers are:
[0084] HD-F (SEQ ID NO. 10):
[0085] CGCGATTCTTAAGGGTGTCCAGTGCGAGGTGCAGCTGGTGGA;
[0086] HD-B55-R (SEQ ID NO.11):
[0087] GCATGGAGGACAGGGCTTGATTGTGGGAGAAGACACTGTCACCTG
[0088] The reaction system is shown in Table 2, and the amplification program is shown in Table 3 below:
[0089] Table 2
[0090]
[0091] table 3
[0092]
[0093] 2. The enzyme digestion system is shown in Table 4. The enzyme digestion temperature is 37°C and the time is 6 hours. The PCR purification kit was used for purification...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com