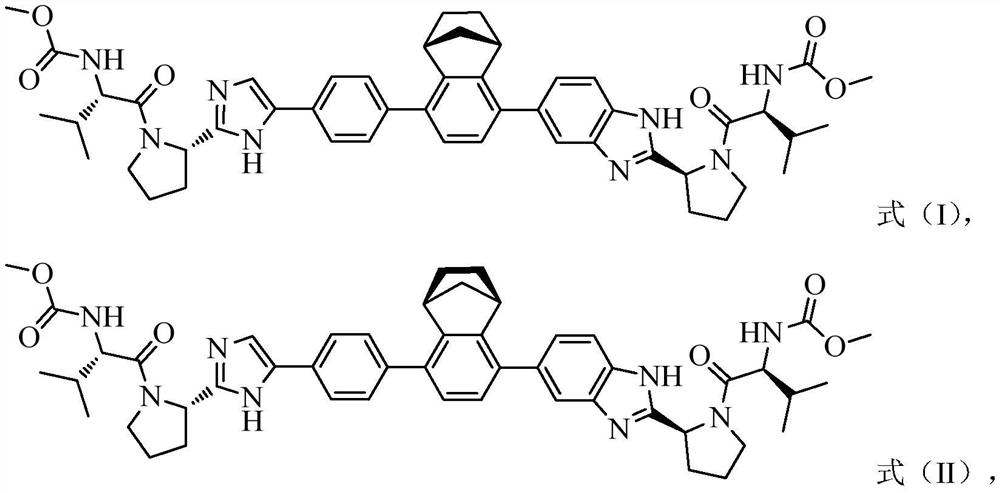
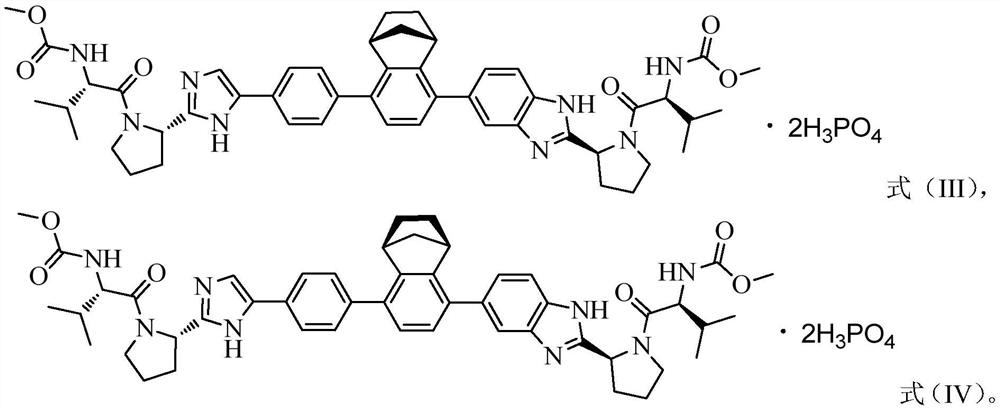
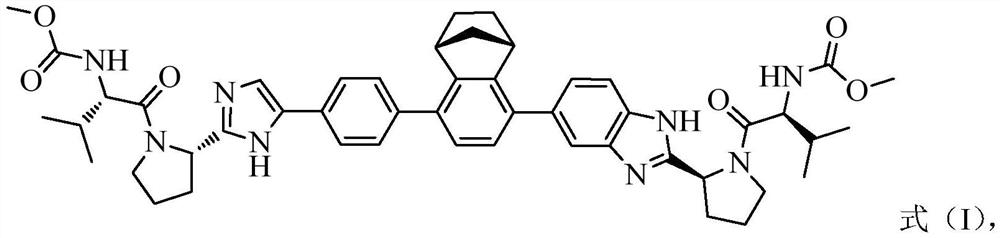
NS5A inhibitor composition
A composition and compound technology, applied in the direction of antiviral agents, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as preclinical research in the early stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Compound III capsules were prepared according to the recipe shown below, and the dissolution rate was determined.
[0161] Preparation:
[0162] (1) The drug particle size D of compound III 50 =47.1μm, microcrystalline cellulose, mannitol, croscarmellose sodium, micropowder silica gel, magnesium stearate passed through a 60-mesh sieve;
[0163] (2) Weigh the components according to the prescription ratio:
[0164] Compound III: 29.27;
[0165] Microcrystalline cellulose: 22.24;
[0166] Mannitol: 44.49;
[0167] Croscarmellose sodium: 2.00;
[0168] Micropowder silica gel: 1.00;
[0169] Magnesium stearate: 1.00;
[0170] Total: 100.
[0171] (3) Mix the microcrystalline cellulose, mannitol, croscarmellose sodium and compound III in the prescribed amount for 20 minutes until uniform, to obtain a mixture;
[0172] (3) The magnesium stearate of the sieved prescription ratio added in the mixture, after total mixing 5min to evenly, is filled into 100mg specificatio...
Embodiment 2
[0175] Compound III capsules were prepared according to the recipe shown below, and the dissolution rate was determined.
[0176] Preparation:
[0177] (1) The drug particle size D of compound III 50 =35.9μm, microcrystalline cellulose, mannitol, croscarmellose sodium, micropowder silica gel, magnesium stearate passed through a 60-mesh sieve;
[0178] (2) Weigh the components according to the prescription ratio:
[0179] Compound III: 29.27;
[0180] Microcrystalline cellulose: 22.24;
[0181] Mannitol: 44.49;
[0182] Croscarmellose sodium: 2.00;
[0183] Micropowder silica gel: 1.00;
[0184] Magnesium stearate: 1.00;
[0185] Total: 100.
[0186] (3) Mix the microcrystalline cellulose, mannitol, croscarmellose sodium and compound III in the prescribed amount for 20 minutes until uniform, to obtain a mixture;
[0187] (3) The magnesium stearate of the sieved prescription ratio added in the mixture, after total mixing 5min to evenly, is filled into 100mg specificatio...
Embodiment 3
[0190] Compound III capsules were prepared according to the recipe shown below, and the dissolution rate was determined.
[0191] Preparation:
[0192](1) The drug particle size D of compound III 50 =22.5μm, microcrystalline cellulose, mannitol, croscarmellose sodium, micropowder silica gel, magnesium stearate passed through a 60-mesh sieve;
[0193] (2) Weigh the components according to the prescription ratio:
[0194] Compound III: 29.27;
[0195] Microcrystalline cellulose: 22.24;
[0196] Mannitol: 44.49;
[0197] Croscarmellose sodium: 2.00;
[0198] Micropowder silica gel: 1.00;
[0199] Magnesium stearate: 1.00;
[0200] Total: 100.
[0201] (3) Mix the microcrystalline cellulose, mannitol, croscarmellose sodium and compound III in the prescribed amount for 20 minutes until uniform, to obtain a mixture;
[0202] (3) The magnesium stearate of the sieved prescription ratio added in the mixture, after total mixing 5min to evenly, is filled into 100mg specification...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com