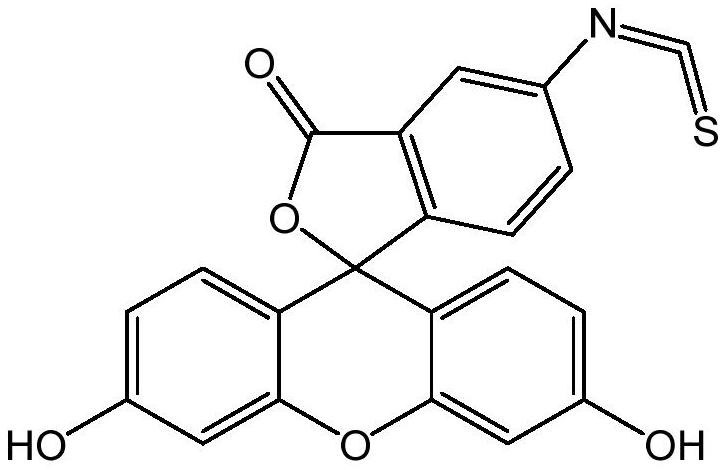
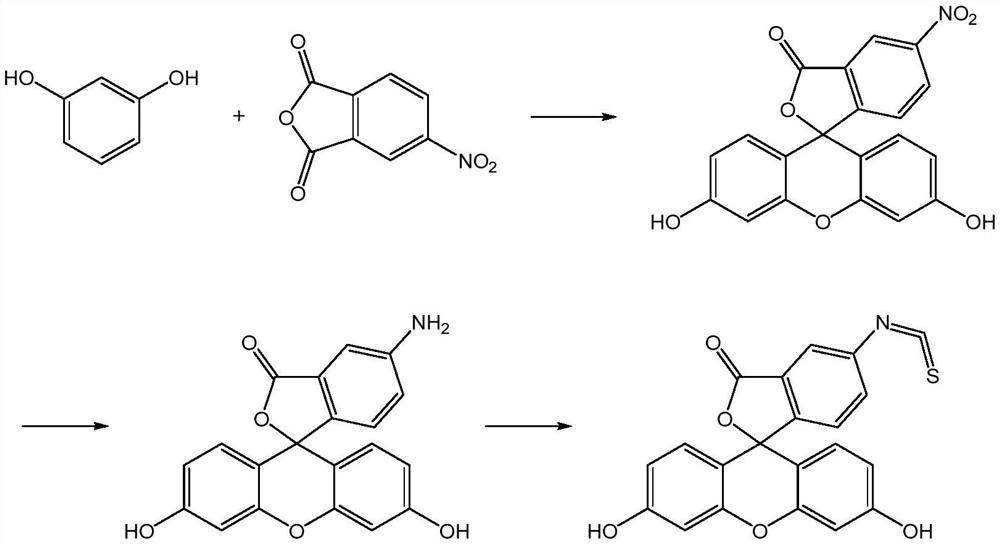
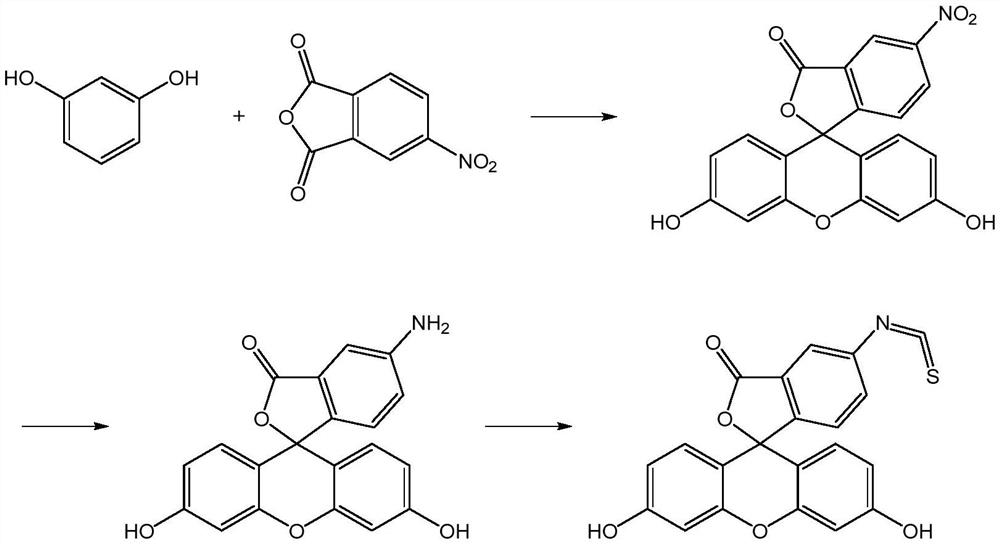
A kind of preparation method of fluorescein probe with specific selectivity
A selective, fluorescein technology, applied in the field of preparation of fluorescein probes, can solve the problems affecting the conversion rate and yield of fluorescein 5-isothiocyanate, and achieve mild conditions, simple operation, improved conversion rate and high efficiency. The effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared by the following preparation method according to the present application:
[0042](1) under the continuous stirring that stirring speed is 40rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 118g, the nitrocyclohexane of 55g and the anhydrous cobalt chloride of 20g are mixed, Heat and melt at 125°C, add 100 g of zinc chloride to it, react at 145°C for 4 hours, then add 1000 mL of hydrochloric acid with a concentration of 0.75 mol / L, heat under reflux for 40 minutes, filter, and filter the filter cake with 55°C Washed with water until neutral, dried at 85°C for 2.5 hours to obtain 192.59 g of product (containing 91.52% 5-nitrofluorescein and 2.37% 6-nitrofluorescein);
[0043] (2) The Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.80 g / mL and 0.15 g / mL, respectively, mix the result of step (1) with 1160 mL of the reducing sol...
Embodiment 2
[0046] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared by the following preparation method according to the present application:
[0047] (1) under the continuous stirring that stirring speed is 50rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 120g, the nitrocyclohexane of 52g and the anhydrous cobalt chloride of 27g are mixed, Heat and melt at 115°C, add 100 g of zinc chloride to it, react at 135°C for 5 hours, then add 1200 mL of hydrochloric acid with a concentration of 0.75 mol / L, heat under reflux for 45 minutes, filter, and filter cake with 50°C Washed with water until neutral, and dried at 80°C for 2 hours to obtain 193.45 g of product (containing 92.38% 5-nitrofluorescein and 2.15% 6-nitrofluorescein);
[0048] (2) The Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.60 g / mL and 0.20 g / mL, respectively, mix the resultant of step (1) with 1350 mL of the reducing solution...
Embodiment 3
[0051] The specific and selective fluorescein probe 5-fluorescein isothiocyanate is prepared by the following preparation method according to the present application:
[0052] (1) under the continuous stirring that stirring speed is 60rpm, the 4-nitrophthalic anhydride of 100g, the resorcinol of 118g, the nitrocyclohexane of 50g and the anhydrous cobalt chloride of 25g are mixed, Heat and melt at 120°C, add 110 g of zinc chloride to it, react at 140°C for 4.5 hours, then add 1000 mL of hydrochloric acid with a concentration of 0.7 mol / L, heat under reflux for 30 minutes, filter, and filter the filter cake with 55°C Washed with water until neutral, dried at 90°C for 3 hours to obtain 193.04 g of product (containing 90.89% 5-nitrofluorescein and 2.89% 6-nitrofluorescein);
[0053] (2) The Na 2 S and Na 2 SO 3 Dissolve in water to prepare reducing solutions with concentrations of 0.80 g / mL and 0.20 g / mL, respectively, mix the result of step (1) with 1160 mL of the reducing sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com