Synthetic method of Jjar mycin
A jaspamycin and synthetic method technology, applied in the field of nucleoside compound synthesis, can solve the problems of long reaction steps and high cost, and achieve the effect of short route steps, cheap reagents and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
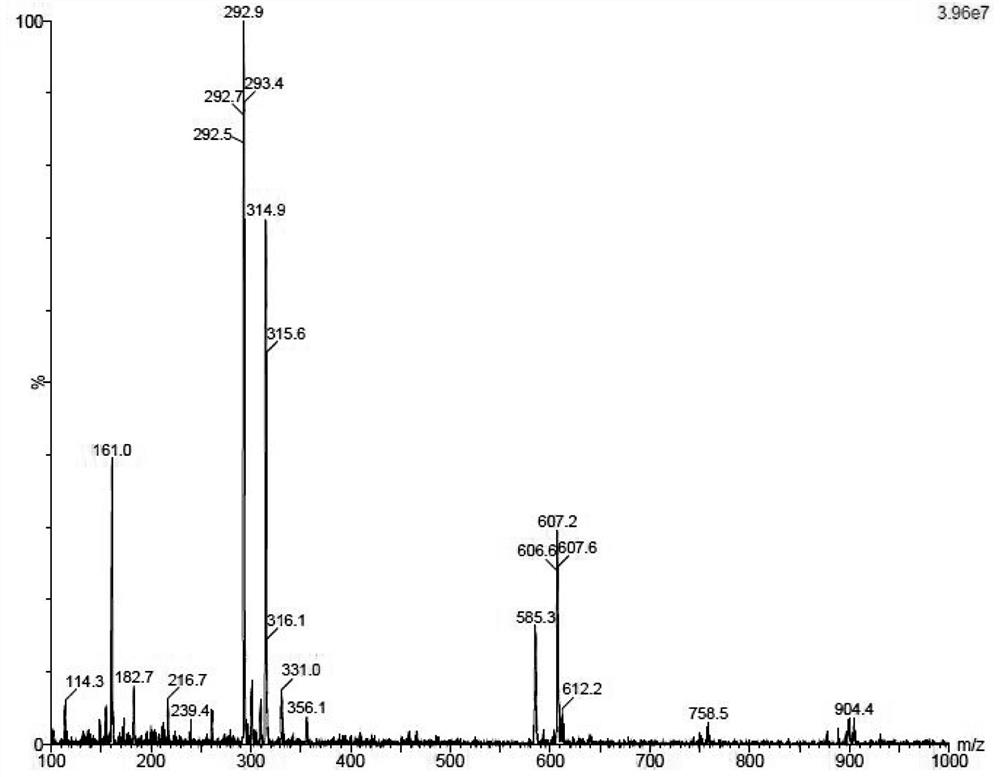
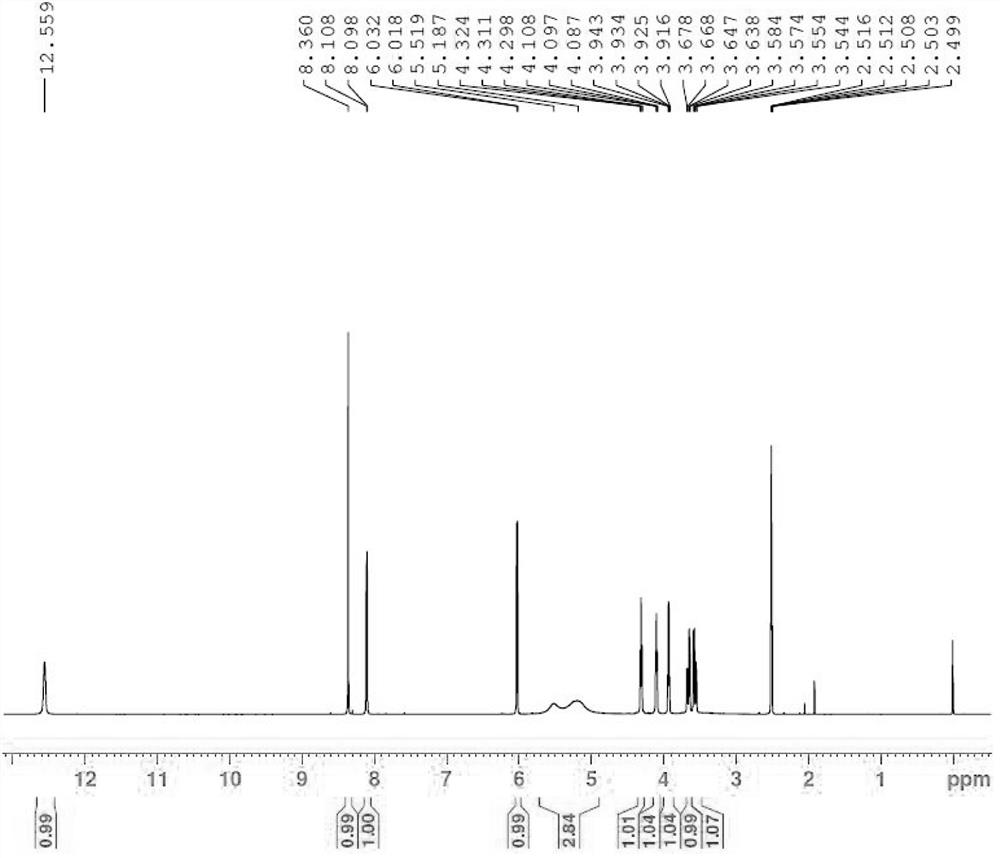
[0014] Example 1: Jaspamycin.
[0015] step 1:
[0016] N,O-bis(trimethylsilyl)acetamide (34 ml, 134 mmol) was added to 4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (34 g , 120 mmol) in a suspension in acetonitrile (1 L). To this was added 1-acetyl-2,3,5-tribenzoyloxy-1-beta-D-ribofuranose (72 g, 140 mmol) and trimethyl trifluoromethanesulfonate after 10 min Silica ester (25.5ml, 134mmol). After the reaction solution was stirred at room temperature for 15 minutes, the temperature was raised to 80° C. for 18 hours. The reaction solution was cooled to room temperature, diluted with ethyl acetate and poured into saturated aqueous sodium bicarbonate solution for liquid separation. The organic phase was washed with brine, dried and concentrated. The resulting residue was slurried in methanol and filtered to obtain compound 1 (79 g, 90% yield) as a white solid.
[0017] Step 2:
[0018] Compound 1 (79 g, 109 mmol) was dispersed in methanol (2 L), sodium methoxide (60 g, 109 mm...
Embodiment 2
[0023] Example 2, the reaction temperature of step 1 is 75°C, and the reaction time is 24 hours; the reaction temperature of step 2 is 30°C, and the reaction time is 12 hours; the step 3 is refluxed for 1 hour, and the reaction temperature of step 4 is 110°C, and the reaction time is 30 hours ; All the other are with embodiment 1.
Embodiment 3
[0024] Example 3, the reaction temperature of step 1 is 85°C, and the reaction time is 12 hours; the reaction temperature of step 2 is 20°C, and the reaction time is 24 hours; the reaction temperature of step 3 is refluxed for 2 hours; the reaction temperature of step 4 is 100°C, and the reaction time is 36 hours. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com