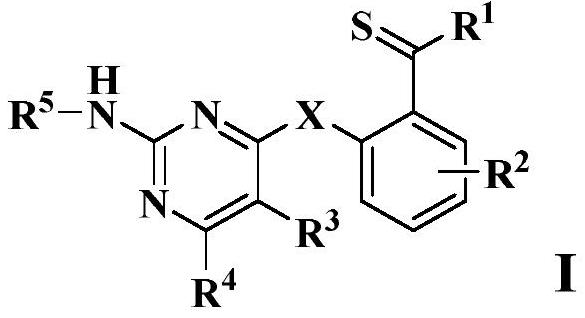
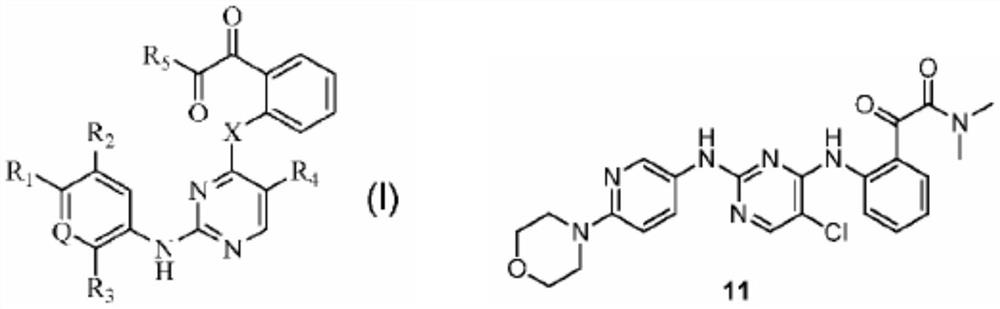
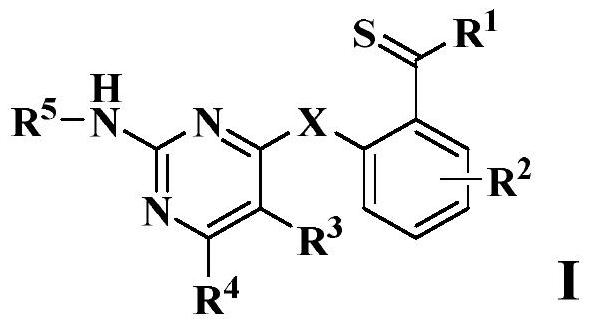
Thiobenzoyl derivative and application thereof
A technology of thiobenzoyl derivatives and alkylthio groups, applied in the field of chemistry, can solve problems such as unreported compounds, and achieve novel structure and excellent effect of inhibiting the activity of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] Embodiment 1: the synthesis of compound 6-121
[0258]
[0259] Add 2-aminothiobenzamide (J.Heterocyclic Chem., 43,1281 (2006)) 3.3g, 2,4,5 trichloropyrimidine 3.6g, sodium carbonate 2.2g, DMF50mL in 100mL flask, Stir and heat at 80°C for 4 hours. After the reaction is detected by TLC, add 150 mL of water, extract with 50 mL of ethyl acetate × 3, combine the organic phases, dry over anhydrous sodium sulfate, and perform column chromatography (eluent is ethyl acetate and petroleum ether ( Boiling range 60-90 ℃), volume ratio is 1:2) purification to obtain 0.53g compound 6-121, 2-((2,5-dichloropyrimidin-4-yl)amino)-N-methylthiobenzene Formamide, yellow solid.
Embodiment 2
[0260] Embodiment 2: the synthesis of compound 7-120
[0261]
[0262] Add 0.53 g of 2-((2,5-dichloropyrimidin-4-yl) amino)-N-methylthiobenzamide, 0.3 g of 4-morpholinoaniline, and p-toluenesulfonate monohydrate into a 100 mL flask 0.3g of acid, 30mL of n-butanol, stirred and heated at 100°C for 4 hours, after the reaction was detected by TLC, add 150mL of water, extract with 50mL of ethyl acetate × 3, combine the organic phases, dry over anhydrous sodium sulfate, column chromatography (elution The solvent is ethyl acetate and petroleum ether (boiling range 60-90° C., volume ratio 2:1) to obtain 0.11 g of compound 7-120 as a gray solid.
[0263] The characterization data of some compounds prepared according to the above preparation method are as follows:
[0264] Compound 7-120:
[0265] 1 H NMR (600MHz, DMSO- d6 )δ10.61(d, J=4.0Hz, 1H), 9.62(s, 1H), 9.16(s, 1H), 8.22(d, J=7.4Hz, 1H), 8.13(s, 1H), 7.48- 7.44(m, 3H), 7.30(d, J=7.5Hz, 1H), 7.19(t, J=7.5Hz, 1H), 6.83(d, J...
Embodiment 3
[0269] Embodiment 3: In vitro detection experiment (MTT method) to cancer cell inhibitory effect is as follows
[0270] Human cancer cell lines used: human ovarian adenocarcinoma cell SK-OV-3, human cervical cancer cell Hela, human non-small cell lung cancer cell A549, and human anaplastic large cell KARPAS299.
[0271] Using in vitro cell culture technology, the inhibitory rate of 5 concentrations of test samples on the growth of human cancer cells was determined by conventional MTT method.
[0272] The cells were taken out from the incubator, washed twice with PBS, digested with 0.25% trypsin solution, added medium to stop the digestion, centrifuged and blown with a pipette to form a cell suspension, and counted under an inverted microscope. Prepare cells into a cell suspension with a concentration of 5x104 / mL, add 100 μL of cells to each well of a 96-well plate, place in 5% carbon dioxide, and culture overnight at 37°C in humidified air, and add the above-mentioned drugs to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com