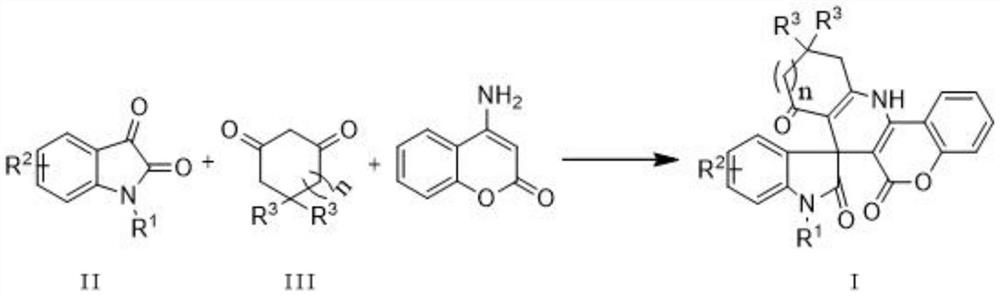
Indole spiropyridino coumarin compound as well as preparation method and application thereof
An indole spiropyridine and coumarin technology, which is applied in the field of pesticides, can solve problems such as no research reports on indole spiropyridine coumarin compounds, and achieves high application potential, high research value, and good bacteriostasis. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
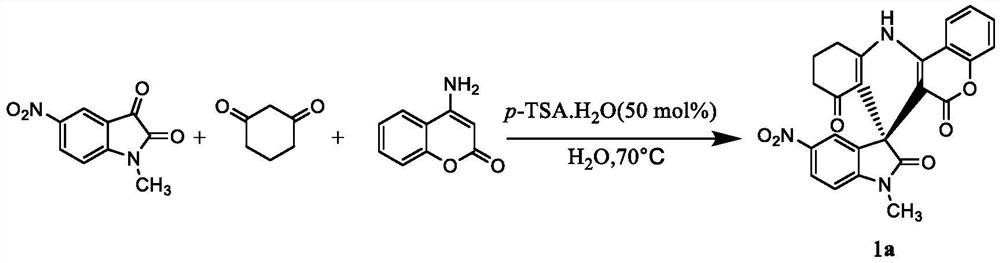
Embodiment 1
[0021]
[0022] Weigh 0.21g (1.0mmol) N-methyl-5-nitroisatin, 0.17g (1.5mmol) 1,3-cyclohexanedione, 0.16g (1.0mmol) 4-aminocoumarin and 0.09g (0.5mmol) p-toluenesulfonic acid monohydrate (p-TSA.H 2 O) dissolved in 5 mL of water, heated to 80° C., stirred for 2 hours, cooled to room temperature, filtered to remove the solvent, dried, and recrystallized from methanol to obtain a yellow solid, that is, a compound having the structure of formula 1a. It was determined that the yield was 81%, m.p.>320°C. 1 H NMR (400MHz, DMSO-d 6 )δ:9.98(s,1H),8.43(dd,J=8.2,1.5Hz,1H),8.18(dd,J=8.6,2.4Hz,1H),7.94(d,J=2.4Hz,1H), 7.74-7.66(m,1H),7.54-7.47(m,1H),7.39(dd,J=8.4,1.2Hz,1H),7.15(d,J=8.7Hz,1H),3.24(s,3H) ,2.94-2.75(m,2H),2.27-2.13(m,2H),2.00-1.85(m,2H).Anal.Calcd for C 24 h 17 N 3 o 6 : C, 65.01; H, 3.86; N, 9.48. Found: C, 64.75; H, 3.95; N, 9.35.
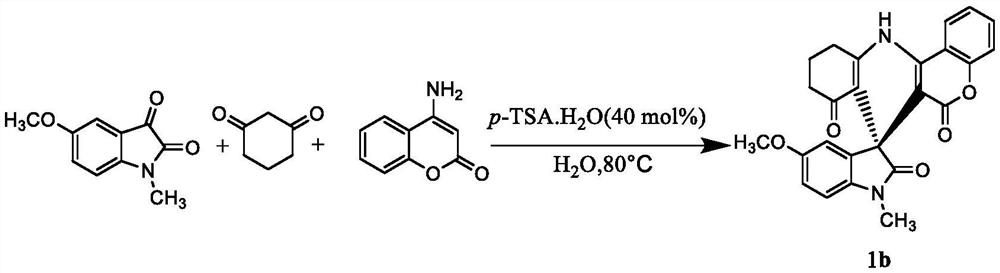
Embodiment 2
[0024]
[0025] Weigh 0.19g (1.0mmol) N-methyl-5-methoxyisatin, 0.22g (2.0mmol) 1,3-cyclohexanedione, 0.16g (1.0mmol) 4-aminocoumarin and 0.08 g(0.4mmol)p-TSA.H 2 O was dissolved in 5 mL of water, heated to 90°C, stirred for 3 hours, cooled to room temperature, filtered to remove the solvent, dried, and recrystallized from a mixed solution of ethyl acetate and ethanol to obtain a yellow solid, namely the compound with the structure of formula 1b. It was determined that the yield was 82%, m.p.301.8-302.4°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 9.74(s, 1H), 8.38(d, J=7.8Hz, 1H), 7.68(t, J=7.6Hz, 1H), 7.47(t, J=7.7Hz, 1H), 7.37(d, J=8.3Hz,1H),6.79-6.69(m,2H),6.63(d,J=2.3Hz,1H),3.62(s,3H),3.10(s,3H),2.87-2.73(m,2H ),2.24-2.09(m,2H),1.97-1.82(m,2H).Anal.Calcd forC 25 h 20 N 2 o 5 H, 4.71; N, 6.54. Found: C, 69.94; H, 4.38; N, 6.28.
Embodiment 3
[0027]
[0028] Weigh 0.19g (1.0mmol) N-methyl-6-chloroisatin, 0.22g (2.0mmol) 1,3-cyclohexanedione, 0.16g (1.0mmol) 4-aminocoumarin and 0.06g ( 0.3mmol)p-TSA.H 2 O was dissolved in 5 mL of 1,2-dichloroethane, heated to 80°C, stirred for 10 hours, cooled to room temperature, filtered to remove the solvent, dried, recrystallized from ethanol and dichloromethane to obtain a yellow solid, which had the structure of formula 1c compound of. It was determined that the yield was 61%, m.p.>320°C. 1 H NMR (400MHz, DMSO-d 6 )δ:9.84(s,1H),8.38(dd,J=8.3,1.5Hz,1H),7.72-7.65(m,1H),7.51-7.45(m,1H),7.38(dd,J=8.4, 1.1Hz, 1H), 7.06-6.97(m, 2H), 6.87(dd, J=7.8, 1.9Hz, 1H), 3.14(s, 3H), 2.87-2.72(m, 2H), 2.26-2.10(m ,2H),1.97-1.81(m,2H).Anal.Calcd for C 24 h 17 ClN 2 o 4 H, 3.96; N, 6.47. Found: C, 66.85; H, 3.70; N, 6.70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com