Polypeptide fragment and medical application thereof
A technology of polypeptide fragments and drugs, which is applied in the field of medicine, can solve problems such as limited effects, and achieve the effects of reducing release rate, improving survival rate, and reducing cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The artificial synthesis method of embodiment 1 peptide segment NYEPPTVVPGGDL
[0018] The peptide chain was synthesized by Fmoc / PyBOP method. The removal of Fmoc protecting group adopts the DMF solution of 30% hexahydropyridine; The cutting off of peptide chain from the resin adopts peptide cutting reagent (trifluoroacetic acid / crystalline phenol / water / thioanisole / ethanedithiol / methyl ethyl sulfide Ether = 81.5 / 5 / 5 / 5 / 2.5 / 1).
[0019] Step 1: Resin treatment before peptide grafting
[0020] i) Weigh 10 g of BOC-Leu-Merrifield resin (purchased from Merck) into a sand core filter reactor.
[0021] ii) add dichloromethane to soak and wash 6 times, 15 ml each time, and filter to remove the washed dichloromethane.
[0022] iii) Add 15 ml of 10% TFA (dichloromethane as solvent) and react at room temperature for 2 hours to remove the BOC protecting group at the N-terminal of the amino acid on the resin.
[0023] iv) add dichloromethane to soak and wash 3 times, 15 millilit...
Embodiment 2
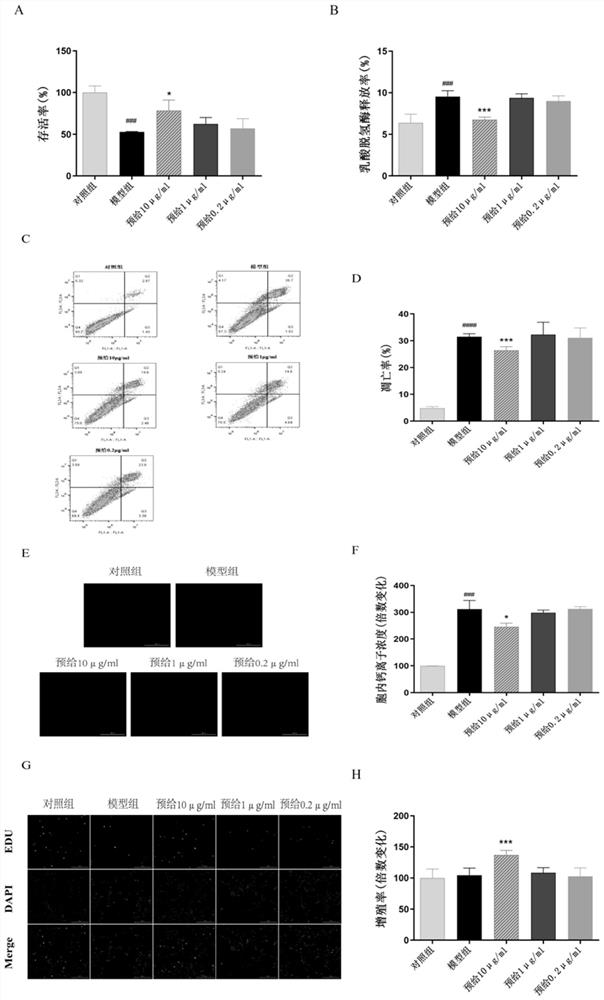
[0053] Example 2 Peptide NYEPPTVVPGGDL in the treatment of Aβ 25-35 Research on nerve cell damage
[0054] Step 1: Experimental grouping (adrenal pheochromoma cells PC12 cells, donated by Mr. Xu Ming, China Pharmaceutical University, n=5)
[0055] i) Control group: spot 96-well plate with 8*10^3 / well, adhere to the wall for 12 hours, change to serum-free medium and culture for 12 hours, then change to fresh serum-free medium and continue to culture for 48 hours.
[0056] ii) Modeling group: spot 96-well plates with 8*10^3 / well, adhere to the wall for 12 hours, replace with serum-free medium and culture for 12 hours, and then replace with freshly prepared Aβ in serum-free medium 25-35 (20μM) to continue to culture for 48h.
[0057] iii) Peptide 10 μg / ml pre-administered group: spot 96-well plates with 8*10^3 / well, adhere to the wall for 12 hours, replace with freshly prepared peptide solution (10 μg / ml) in serum-free medium and incubate for 12 hours, Replace with freshly pre...
Embodiment 3
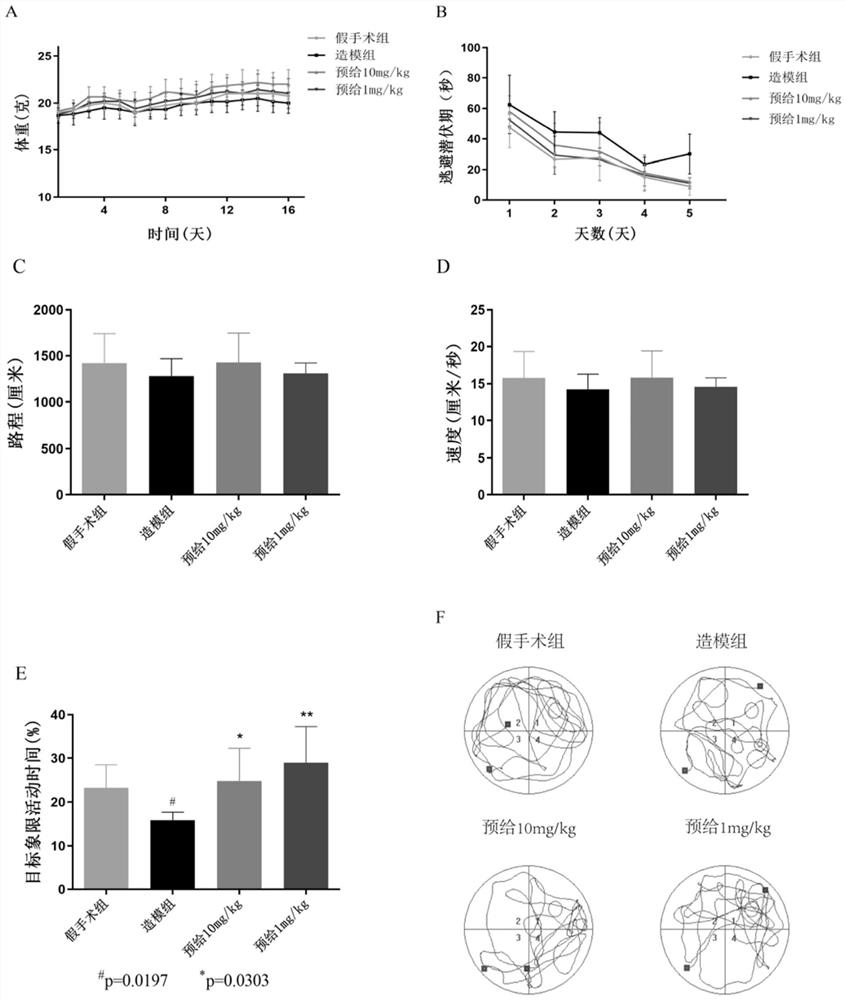
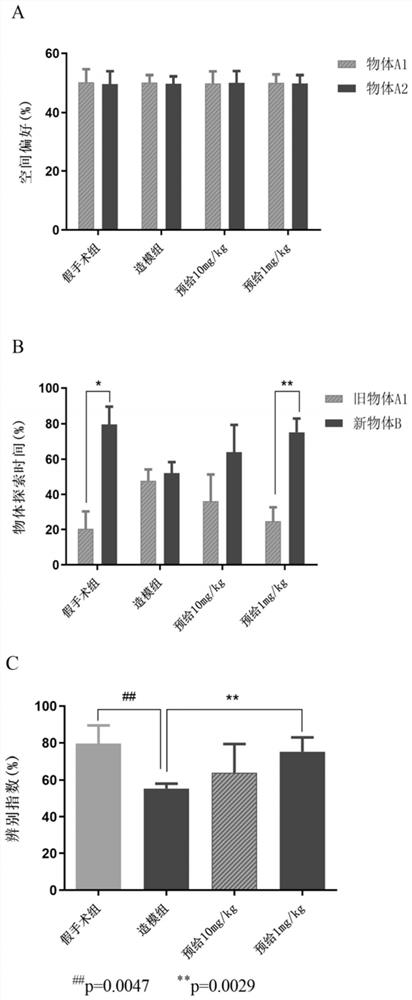
[0085] Example 3 Peptide NYEPPTVVPGGDL in the treatment of Aβ 25-35 Study on memory dysfunction
[0086] Step 1: Experimental grouping (6-week-old C57 male mice, purchased from the Comparative Medicine Center of Yangzhou University, n=6)
[0087] i) Sham operation group: 4 days pre-administration of equal volume of normal saline (0.1ml / 20g). After the sham operation, an equal volume of normal saline (0.1ml / 20g) was injected intraperitoneally.
[0088] ii) Modeling group: 4 days pre-administration of equal volume of normal saline (0.1mL / 20g). After modeling, an equal volume of saline (0.1 mL / 20 g) was injected intraperitoneally.
[0089] iii) Peptide 10 mg / kg pre-administration group: peptide NYEPPTVVPGGDL (10 mg / kg*day) was pre-administered for 4 days. After modeling, an equal volume of peptide NYEPPTVVPGGGSH (0.1 mL / 20 g) was injected intraperitoneally.
[0090] iv) Peptide 1 mg / kg pre-administration group: 4-day pre-administration of peptide NYEPPTVVPGGDL (1 mg / kg*day)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com