Method for synthesizing On-DNA dihydropyrazole compound
A technology for dihydropyrazoles and compounds, applied in the field of coding compound libraries, can solve the problems of no reports, etc., and achieve the effects of simple operation, single product, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
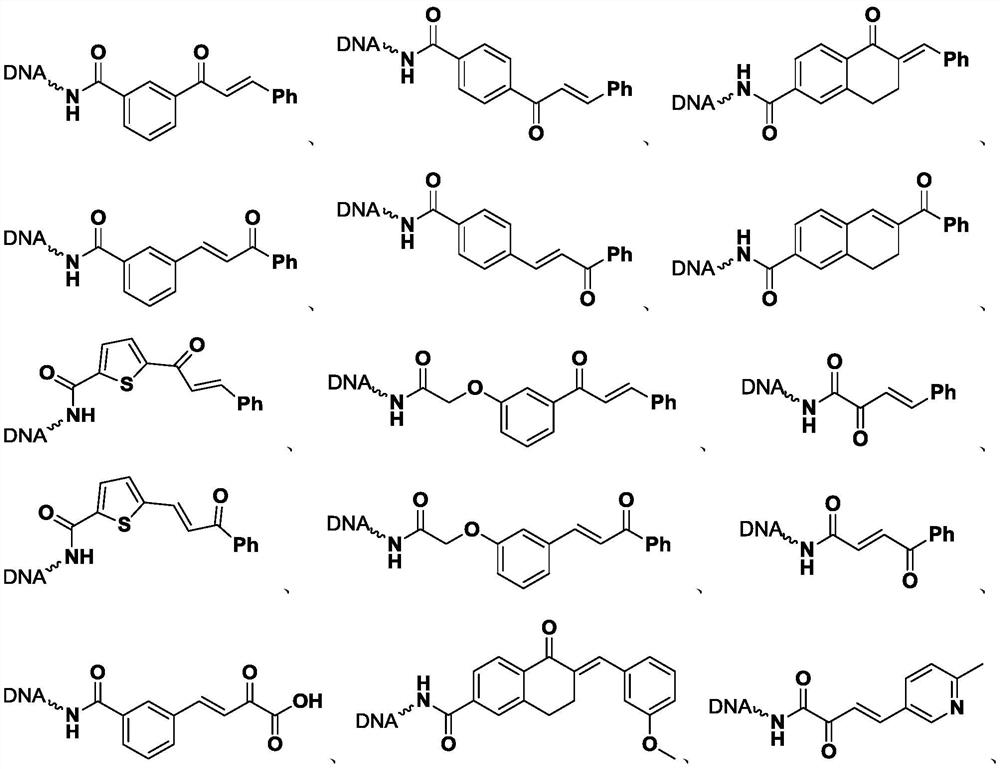
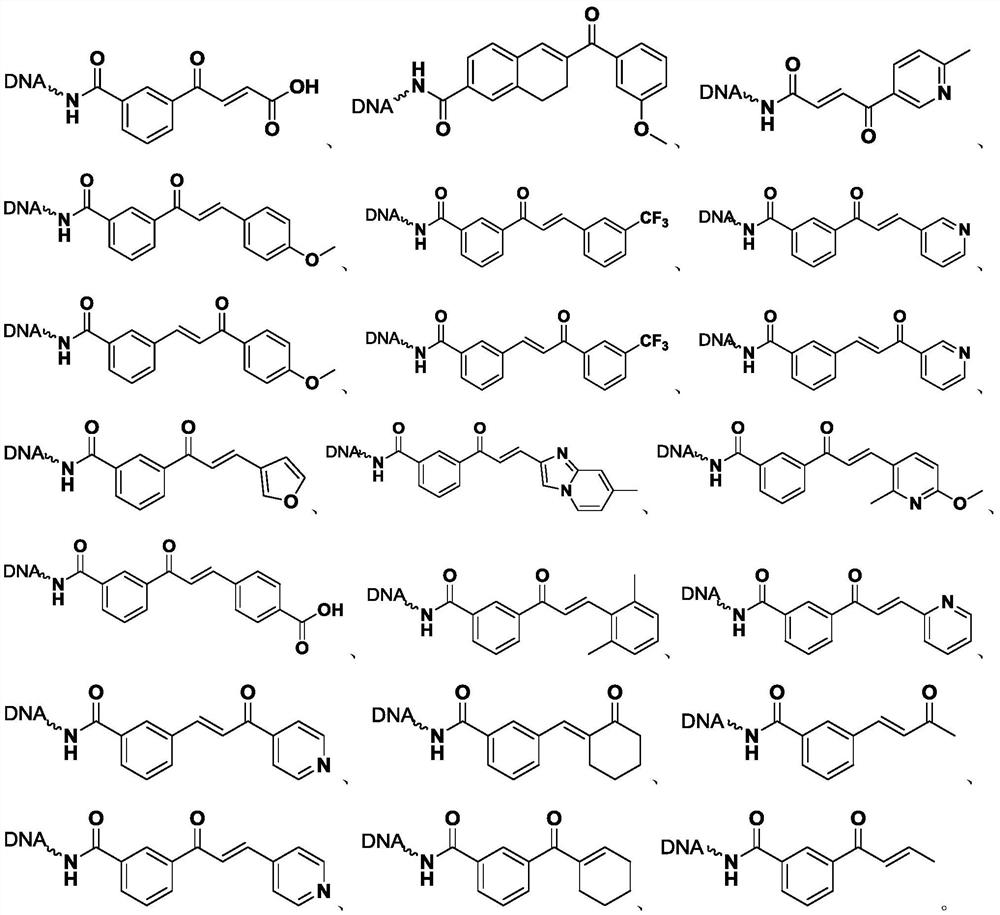
[0054] The synthesis of embodiment 1, On-DNA dihydropyrazole compound
[0055] Step 1. Synthesis of On-DNA α, β-unsaturated carbonyl compounds
[0056]
[0057] Dissolve the On-DNA arylethanone compound (1) in 250 mM, pH=9.4 boric acid buffer solution to prepare a 1 mM concentration solution (20 μL, 20 nmol), and add benzaldehyde (4000 nmol, 200 equivalents, 200mM DMSO), potassium hydroxide (10000nmol, 500 equivalents, 500mM double distilled water), mix well, and react at 30°C for 1 hour.
[0058] After the reaction is completed, carry out ethanol precipitation: add a total volume of 10% 5M sodium chloride solution to the reacted solution, and then continue to add 3 times the total volume of absolute ethanol, shake evenly, and place the reaction in dry ice to freeze After 0.5 hours, centrifuge at 12000rpm for half an hour, discard the supernatant, and dissolve the remaining precipitate with deionized water to obtain a solution of On-DNA α, β-unsaturated carbonyl compound (...
Embodiment 2
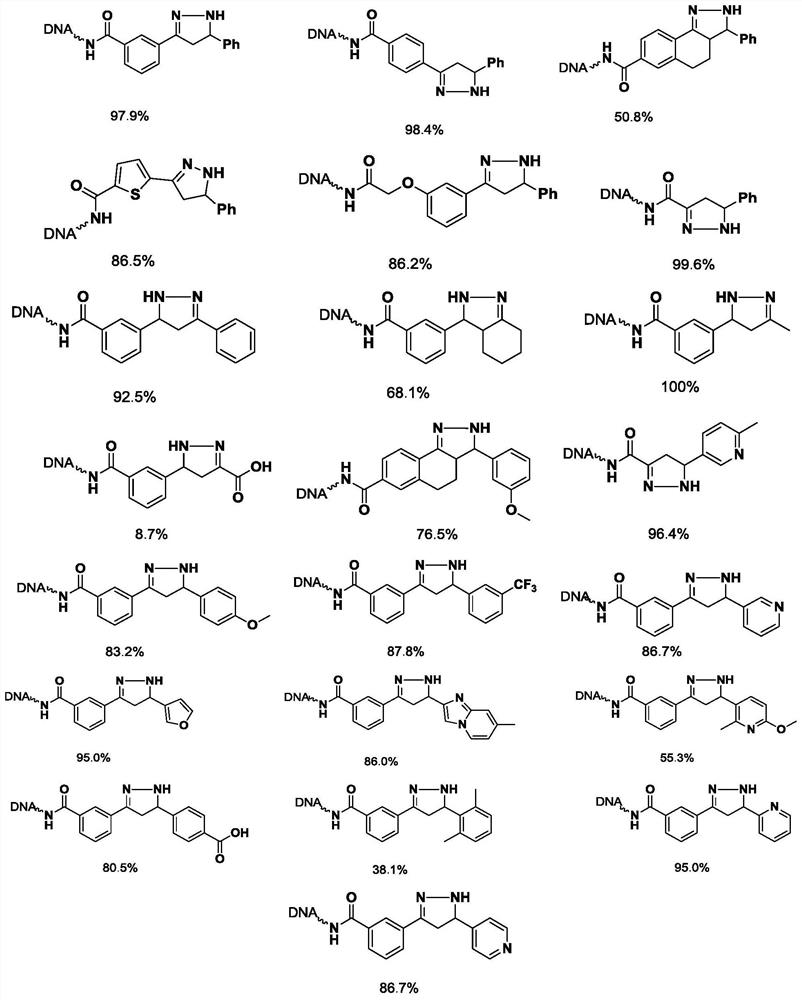
[0063] Embodiment 2, the synthesis of On-DNA dihydropyrazole compound
[0064]
[0065] Dissolve 22 kinds of On-DNA α, β-unsaturated carbonyl compounds in 250mM, pH=9.4 boric acid buffer solution respectively to prepare 1mM concentration solution (20μL, 20nmol), add hydrazine hydrate (4000nmol, 200 equivalents) to the solution , 200mM double-distilled water), mix well, and react at 30°C for 1 hour.
[0066] After the reaction is completed, carry out ethanol precipitation: add a total volume of 10% 5M sodium chloride solution to the reacted solution, and then continue to add 3 times the total volume of absolute ethanol, shake evenly, and place the reaction in dry ice to freeze After 0.5 hours, centrifuge at 12,000rpm for half an hour, pour off the supernatant, and dissolve the remaining precipitate with deionized water to obtain a solution of On-DNA product. After quantifying the OD with a microplate reader, send it to LCMS to confirm the reaction conversion Rate.
[0067]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com