Lysosome targeting fluorescent probe and synthesis method and cell imaging application thereof
A technology of fluorescent probes and synthesis methods, applied in the field of fluorescent probes, can solve the problems of low penetration depth, poor photostability, poor water solubility, etc., and achieve the effect of high spatial resolution and low background signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
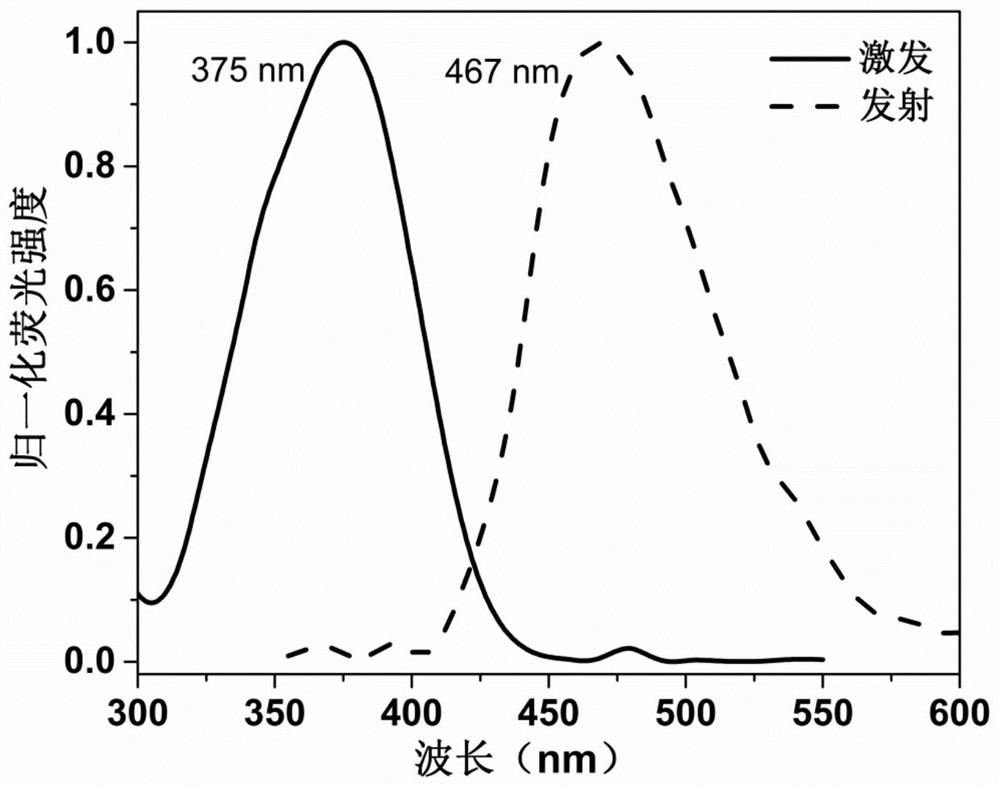
[0040] Synthesis of lysosomal probe N-2-morpholinoethyl-1,8-naphthalimide (Lyso-405).
[0041] Synthesis of intermediate N-(2-morpholinyl)ethyl-1,8-naphthalimide (Lyso-Br):
[0042]
[0043] Dissolve 4-bromo-1,8-naphthalene anhydride (277.07mg, 1.00mmol) in 13.85mL ethanol, then add 0.03mL (0.21mmol) N-(2-aminoethyl)morpholine to the reaction solution . The reaction solution was heated to 60°C and stirred for 0.5h. After cooling down, the off-white solid was filtered with suction and washed with ethanol, and dried in vacuo to obtain 56.40 mg of N-(2-morpholinyl)ethyl-1,8-naphthoimide with a yield of 69%.
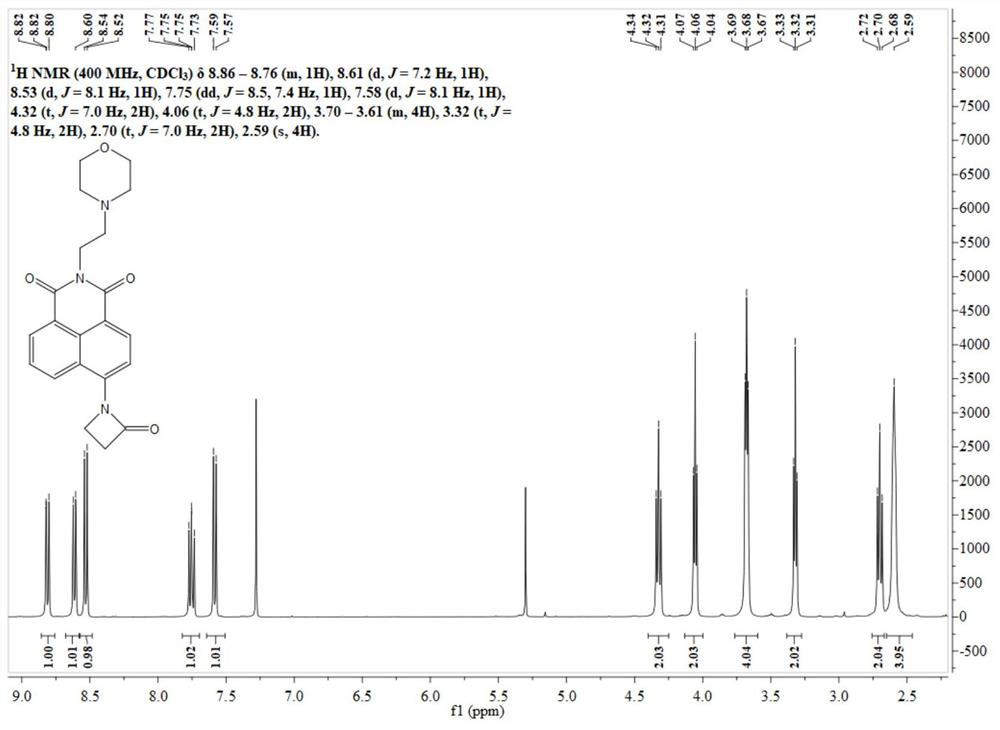
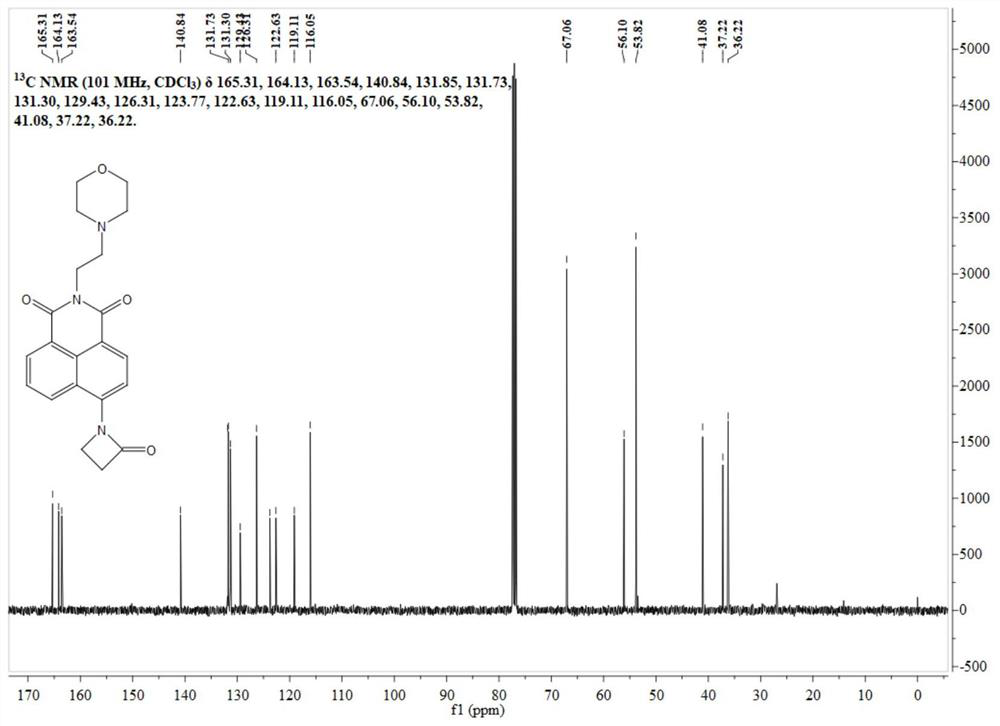
[0044] 1 H NMR (400MHz, CDCl 3 )δ8.65(d, J=8.0Hz, 1H), 8.58(d, J=8.0Hz, 1H), 8.11(d, J=8.0Hz, 1H), 8.05(d, J=8.0Hz, 1H) ,7.86(t,J=8.0Hz,1H),4.34(t,J=7.2Hz,2H),3.68(br,4H),2.72(br,2H),2.60(br,4H).
[0045] Synthesis of Probe N-2-Morpholinylethyl-1,8-Naphthimide (Lyso-405)
[0046]
[0047] N-(2-morpholinyl)ethyl-1,8-naphthalimide (50.60mg, 0.13mmol), 2-azetidinon...
Embodiment 2
[0053] Synthesis of lysosomal probe N-2-morpholinoethyl-1,8-naphthalimide (Lyso-405).
[0054] Synthesis of intermediate N-(2-morpholinyl)ethyl-1,8-naphthalimide (Lyso-Br):
[0055]
[0056] Dissolve 4-bromo-1,8-naphthalene anhydride (277.07mg, 1.00mmol) in 22.17mL ethanol, then add 0.084mL (0.64mmol) N-(2-aminoethyl)morpholine to the reaction solution . The reaction solution was heated to 100°C and stirred for 2h. After cooling down, the off-white solid was suction-filtered and washed with ethanol, and dried in vacuo to obtain 164.41 mg of N-(2-morpholinyl)ethyl-1,8-naphthoimide with a yield of 66%.
[0057] Synthesis of Probe N-2-Morpholinylethyl-1,8-Naphthimide (Lyso-405)
[0058]
[0059] N-(2-morpholinyl)ethyl-1,8-naphthalimide (50.60mg, 0.13mmol), 2-azetidinone (35.42mg, 0.50mmol), Cs 2 CO 3 (162.91mg, 0.50mmol), G3-Xantphos (XantPhos Pd G3 95%) (1mol%) was placed in a two-necked bottle and replaced with nitrogen 3-4 times. Add 4.05 mL of dry dioxane to the r...
Embodiment 3
[0062] Synthesis of lysosomal probe N-2-morpholinoethyl-1,8-naphthalimide (Lyso-405).
[0063] Intermediate N-(2-morpholinyl)ethyl-1,8-naphthalimide (Lyso-Br):
[0064]
[0065] Dissolve 4-bromo-1,8-naphthalene anhydride (277.07mg, 1.00mmol) in 30.48mL ethanol, then add 0.14mL (1.06mmol) N-(2-aminoethyl)morpholine to the reaction solution . The reaction solution was heated to 140°C and stirred for 4h. After cooling down, the off-white solid was suction-filtered and washed with ethanol, and dried in vacuo to obtain 284.15 mg of N-(2-morpholinyl)ethyl-1,8-naphthoimide with a yield of 73%.
[0066] Synthesis of Probe N-2-Morpholinylethyl-1,8-Naphthimide (Lyso-405)
[0067]
[0068] N-(2-morpholinyl)ethyl-1,8-naphthalimide (50.60mg, 0.13mmol), 2-azetidinone (50.60mg, 0.71mmol), Cs 2 CO 3 (231.33 mg, 0.71 mmol), G3-Xantphos (XantPhos Pd G3 95%) (1 mol%) was placed in a two-necked bottle and replaced with nitrogen 3-4 times. 5.06 mL of dry dioxane was added to the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com