Antigen-specific Treg as well as preparation method and application thereof
A specific and antigen-specific technology, applied in the field of antigen-specific Treg and its preparation, can solve the problems of inapplicability to large-scale clinical application, complex preparation process of antigen peptide, high production cost, etc., to induce long-term immune tolerance and save labor costs Compared with preparation cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
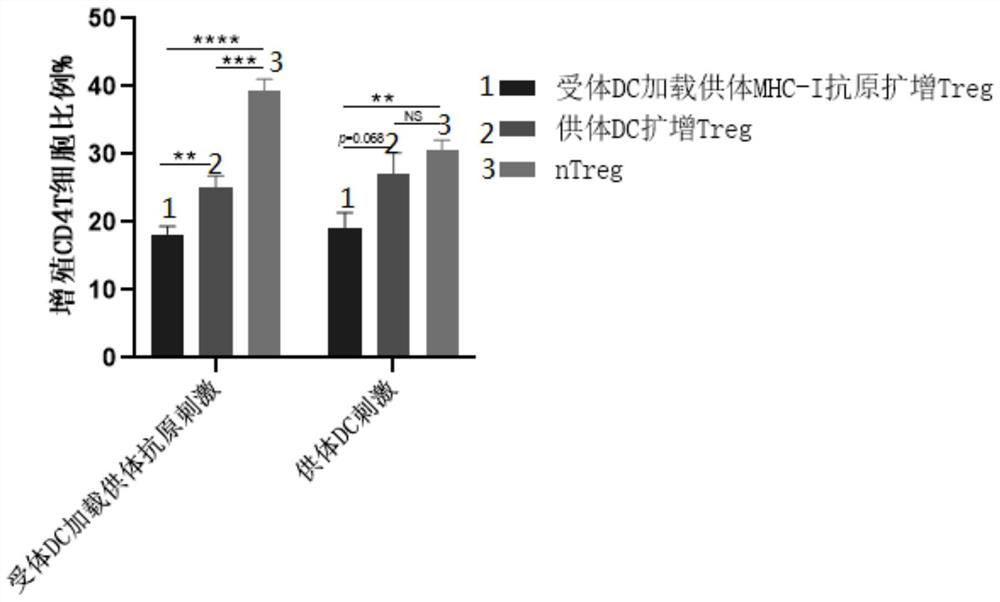
[0042] This embodiment provides a method for preparing antigen-specific Treg, which includes the following steps:
[0043] S1. On day 0, collect monocytes from the peripheral blood of recipients, culture them at 37°C for 1 hour, remove the medium, remove non-adherent cells, or sort mononuclear cells by magnetic bead sorting and flow cytometry. Nucleated cells were added to a fresh medium containing GM-CSF at a concentration of 100 ng / mL and IL-4 at a concentration of 20 ng / mL and cultured for 24 hours to obtain the first culture supernatant;
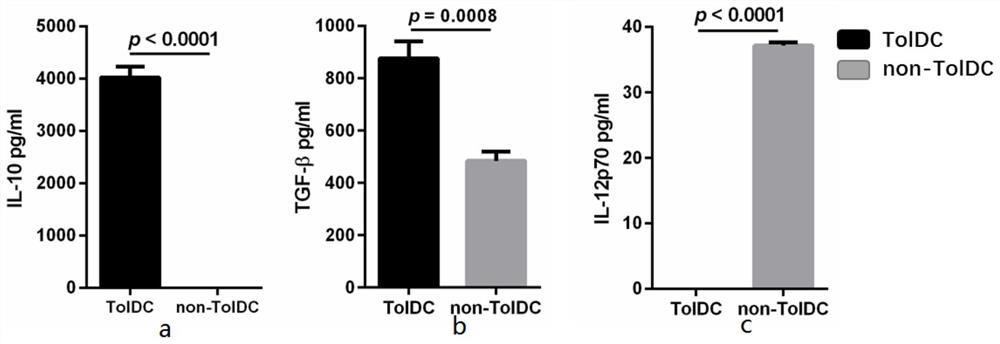
[0044] On the first day, 1 ng / mL of TGF-β1 and 20 ng / mL of IL-10 were added to the above-mentioned first culture supernatant and cultured for 24 hours to obtain the second culture supernatant;
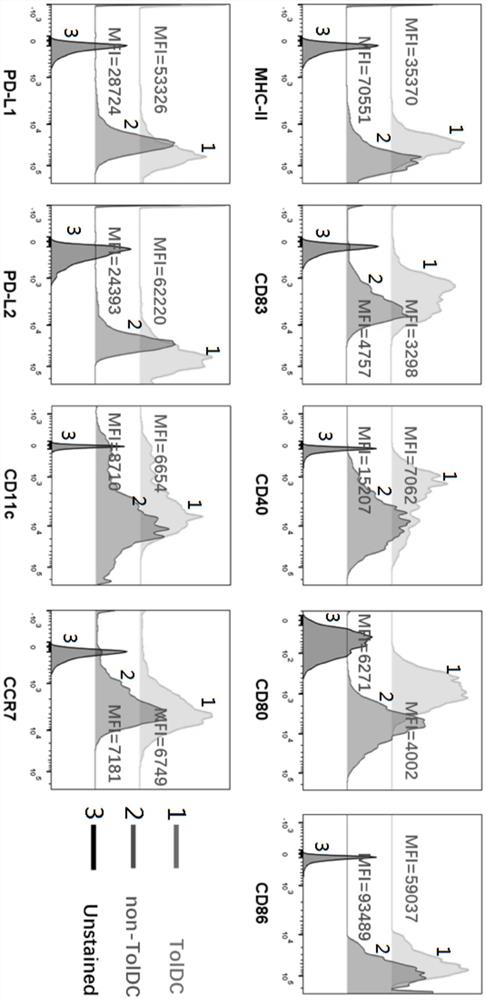
[0045] On the second day, the tolDC cytokine cocktail was added to the second culture supernatant and cultured for 24 hours to induce the maturation of the tolDC to obtain the receptor tolDC. Among them, the tolDC cytokine cocktail includes the ...
Embodiment 2
[0052] This embodiment provides a method for preparing antigen-specific Treg, which includes the following steps:
[0053] S1. On day 0, collect monocytes from the peripheral blood of recipients, culture them at 37°C for 1 hour, remove the medium, remove non-adherent cells, or sort mononuclear cells by magnetic bead sorting and flow cytometry. Nucleated cells were added to a fresh medium containing GM-CSF at a concentration of 80 ng / mL and IL-4 at a concentration of 15 ng / mL and cultured for 24 hours to obtain the first culture supernatant;
[0054] On the first day, 0.5 ng / mL of TGF-β1 and 15 ng / mL of IL-10 were added to the above-mentioned first culture supernatant and cultured for 24 hours to obtain the second culture supernatant;
[0055] On the second day, the tolDC cytokine cocktail was added to the second culture supernatant and cultured for 24 hours to induce the maturation of the tolDC to obtain the receptor tolDC. Among them, the tolDC cytokine cocktail includes the...
Embodiment 3
[0062] This embodiment provides a method for preparing antigen-specific Treg, which includes the following steps:
[0063] S1. On the 0th day, collect the mononuclear cells from the peripheral blood of the recipient, culture them at 37°C for 1 hour, remove the medium, remove the non-adherent cells or sort out the mononuclear cells by magnetic bead sorting and flow cytometric sorting. Nucleated cells were added to a fresh medium containing GM-CSF at a concentration of 120 ng / mL and IL-4 at a concentration of 25 ng / mL and cultured for 24 hours to obtain the first culture supernatant;
[0064] On the first day, 1.5 ng / mL of TGF-β1 and 25 ng / mL of IL-10 were added to the above-mentioned first culture supernatant and cultured for 24 hours to obtain the second culture supernatant;
[0065] On the second day, the tolDC cytokine cocktail was added to the second culture supernatant and cultured for 24 hours to induce the maturation of the tolDC to obtain the receptor tolDC. Among them...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com