Application of fluorescent dye with intramolecular switch in super-resolution imaging
A technology of super-resolution imaging and fluorescent dyes, applied in organic dyes, analytical materials, luminescent materials, etc., can solve the problems of photobleaching and can not meet the long-term imaging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
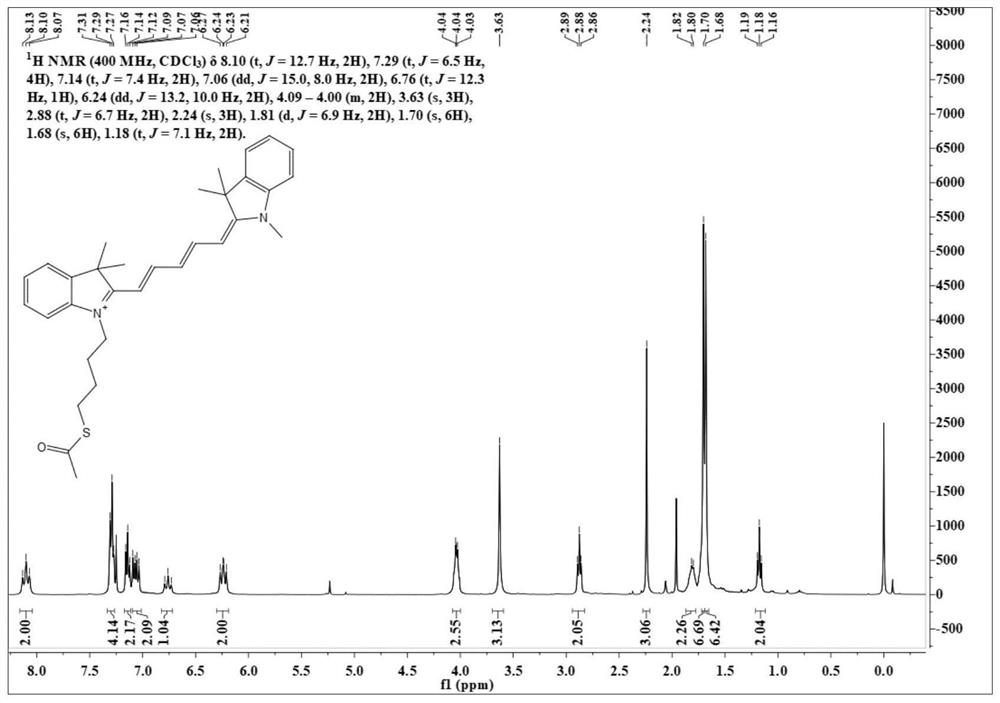
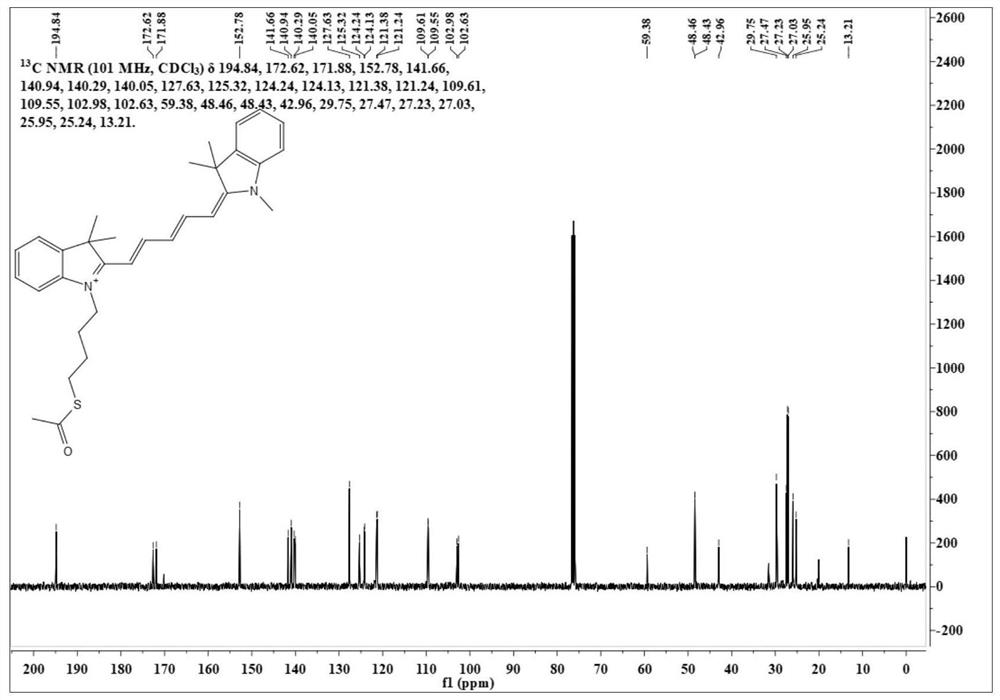
[0051] Synthesis of Dye CyS-4C
[0052] The synthetic route and product structure of the intermediate hemicyanine are as follows:
[0053]
[0054] Weigh N-methyl-2,3,3-trihydroindole (300mg, 1mmol) and malondialdehyde derivatives (284mg, 1mmol) into a single-necked bottle, add solvent acetic anhydride 3mL, heat up to 90°C and stir 2 hours. The solvent was removed under reduced pressure, and silica gel column chromatography (dichloromethane / methanol=50 / 1, V / V) gave 181 mg of a reddish-brown solid with a yield of 60%.
[0055] Its high-resolution mass spectrometry data are as follows:
[0056] HRMS (ESI): m / z: [M] + : Calculated: 345.1961, Experimented: 345.1964.
[0057] After testing, its structure is shown in the above formula.
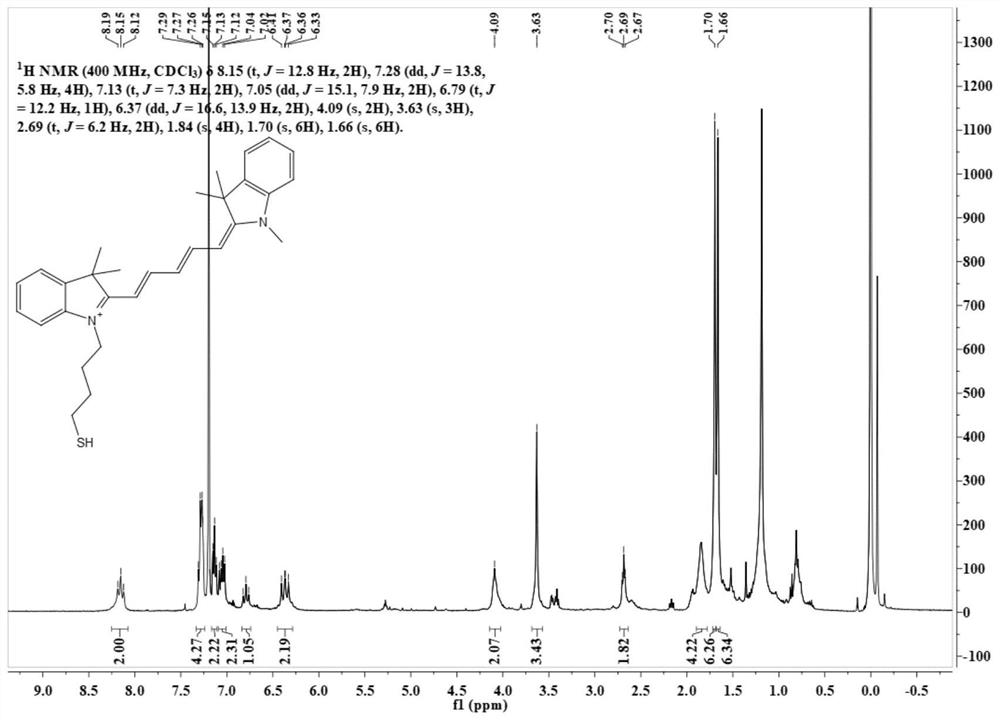
[0058] Synthesis of Intermediate N-thioacetylbutyl-2,3,3-trimethylindoline
[0059]
[0060] Weigh the compound N-(4-bromobutyl)-2,3,3-trimethyltrihydroindole (150mg, 0,4mmol), potassium thioacetate (46mg, 0.4mmol) into a round bottom fl...
Embodiment 2
[0088] Synthesis of Dye CyS-3C
[0089] The synthetic route and product structure of the intermediate hemicyanine are as follows:
[0090]
[0091] Weigh N-methyl-2,3,3-trihydroindole (300mg, 1mmol) and malondialdehyde derivatives (312mg, 1mmol) into a single-necked bottle, add solvent acetic anhydride 3mL, heat up to 110°C and stir 1 hour. The solvent was removed under reduced pressure, and silica gel column chromatography (dichloromethane / methanol=50 / 1, V / V) gave 181 mg of a reddish-brown solid with a yield of 60%.
[0092] Its high-resolution mass spectrometry data are as follows:
[0093] HRMS (ESI): m / z: [M] + : Calculated: 345.1961, Experimented: 345.1964.
[0094] After testing, its structure is shown in the above formula.
[0095] Synthesis of Intermediate N-thioacetylpropyl-2,3,3-trimethyltrihydroindole
[0096]
[0097] Weigh the compound N-(3-bromopropyl)-2,3,3-trimethyltrihydroindole (300mg, 0.8mmol), and potassium thioacetate (1.14mg, 10mmol) into a ro...
Embodiment 3
[0119] Synthesis of Dye CyS-2C
[0120] The synthetic route and product structure of the intermediate hemicyanine are as follows:
[0121]
[0122] Weigh N-methyl-2,3,3-trihydroindole (300mg, 1mmol) and malondialdehyde derivatives (284mg, 1mmol) into a single-necked bottle, add solvent acetic anhydride 3mL, heat up to 110°C and stir 2 hours. The solvent was removed under reduced pressure, and silica gel column chromatography (dichloromethane / methanol=50 / 1, V / V) gave 181 mg of a reddish-brown solid with a yield of 60%.
[0123] Its high-resolution mass spectrometry data are as follows:
[0124] HRMS (ESI): m / z: [M] + : Calculated: 345.1961, Experimented: 345.1964.
[0125] After testing, its structure is shown in the above formula.
[0126] Synthesis of intermediate 2-thioacetyl-bromoethane
[0127]
[0128] The compound 1,2-dibromoethane (500 μL, 5.8 mmol) was dissolved in N,N-dimethylacetamide to form a solution (S1), and the compound potassium thioacetate (726 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com