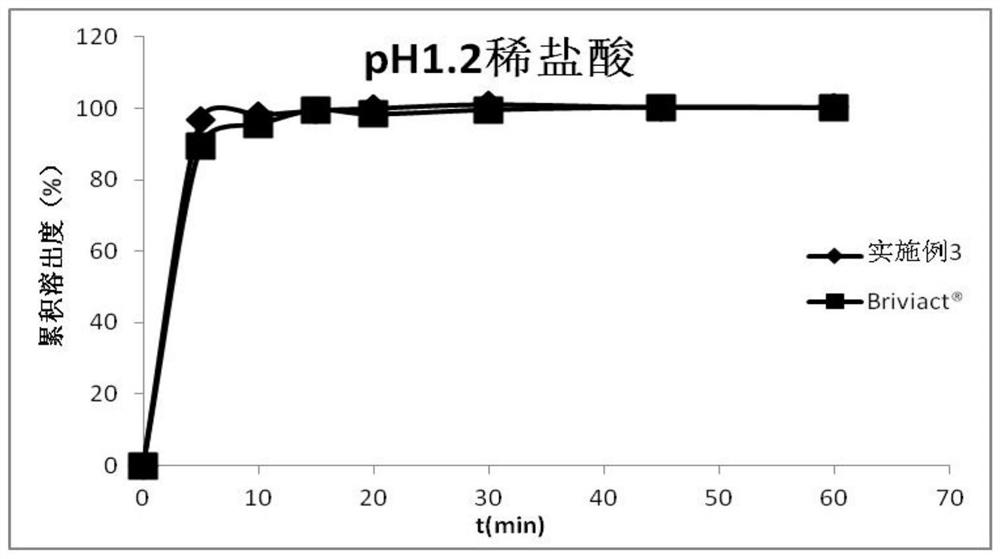
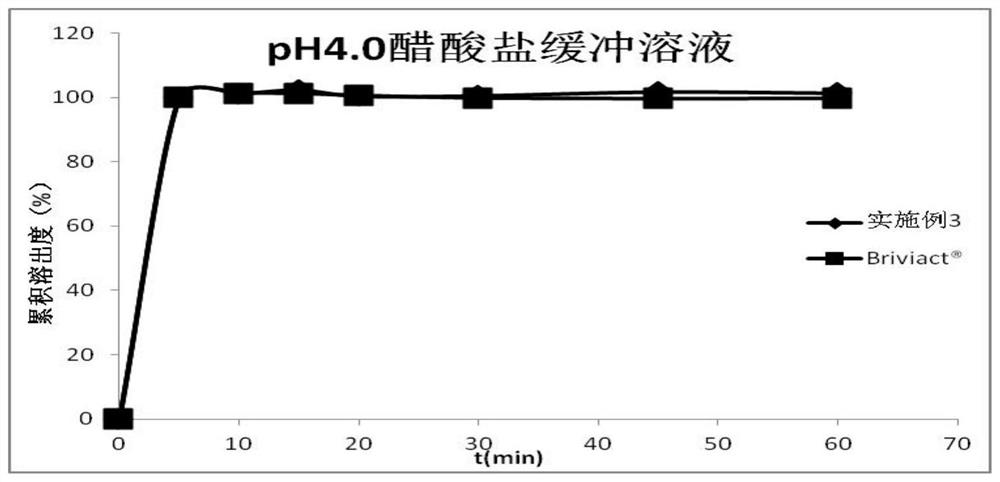
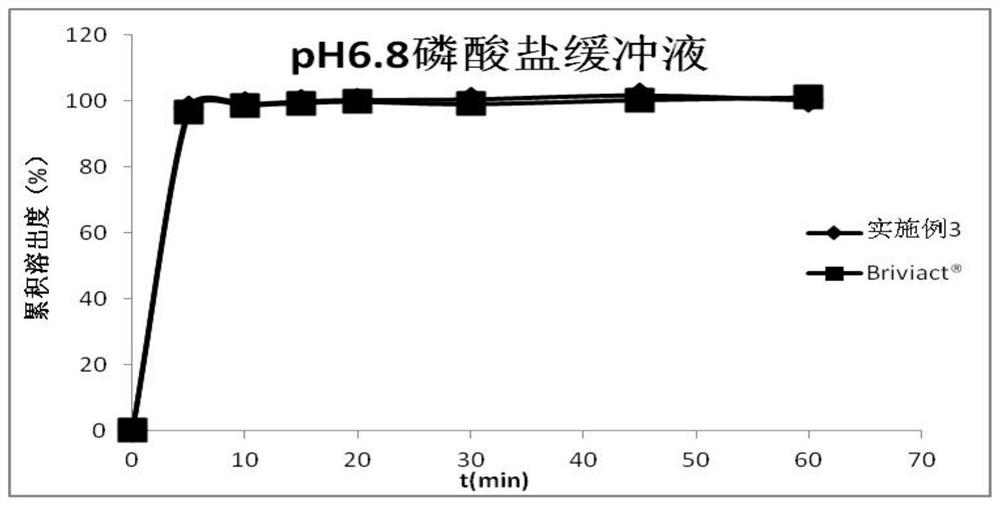
Oral instant film preparation containing 2-oxo-1-pyrrolidine derivative and preparation method and application thereof
A technology of oral instant film and derivatives, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, drug combinations, etc., can solve the problems of limited application functions and decreased mechanical properties of instant films. Achieve the effect of reducing clinical care costs, low cost, and short disintegration time limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The present invention also provides a preparation method of an oral instant film preparation comprising 2-oxo-1-pyrrolidine derivatives, comprising the following steps:
[0076] S1, dispersing a predetermined amount of highly water-soluble film-forming material into water at 70-90°C, stirring and dissolving to obtain a solution of highly water-soluble film-forming material, and then cooling to room temperature for later use;
[0077] S2, adding a predetermined proportion of active ingredients 2-oxo-1-oxolidine derivatives, plasticizers, stabilizers, flavoring agents and opacifiers into the highly water-soluble film-forming material solution, and stirring for 3 to 8 minutes ; Then high-speed shearing homogenization treatment to obtain a mixed solution; the steps of the high-speed shearing homogenization treatment are: the high-speed shearing speed is set to 5000-20000rpm, and the shearing homogenization time is 3-10min;
[0078] S3, after the mixed solution obtained in s...
Embodiment 1
[0087] An oral instant film preparation containing 2-oxo-1-pyrrolidine derivatives is prepared through the following prescription composition and preparation ratio. (Single dose total weight: 80mg, batch size: 1000 tablets)
[0088] name Dosage (g) Ratio (w / w, %) Bovaracetam 10 12.5 Hydroxypropylmethylcellulose 66.3 82.875 polyethylene glycol 2 2.5 Tea polyphenols 1 1.25 Sucralose 0.2 0.25 Titanium dioxide 0.5 0.625 purified water Add water to 1000mL /
[0089] This preparation method comprises the steps:
[0090] S1, disperse the highly water-soluble film-forming material into water at 85°C, stir and dissolve to obtain a highly water-soluble film-forming material solution, and then cool it to room temperature for later use;
[0091] S2, according to the preparation ratio in the above table, add the active ingredient 2-oxo-1-rolidine derivative, plasticizer polyethylene glycol, stabilizer tea polyphenol, flav...
Embodiment 2
[0094] The oral instant film preparation containing 2-oxo-1-pyrrolidine derivatives is prepared by using the above preparation method through the following preparation ratio. (Single dose total weight: 80mg, batch size: 1000 tablets)
[0095]
[0096]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com