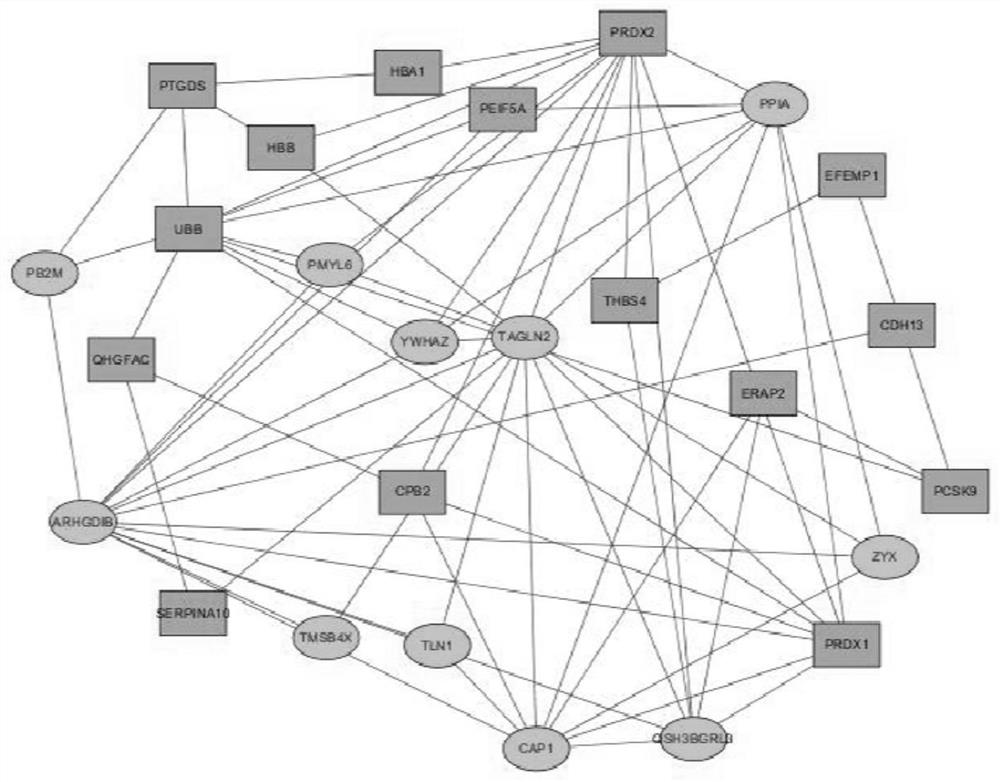
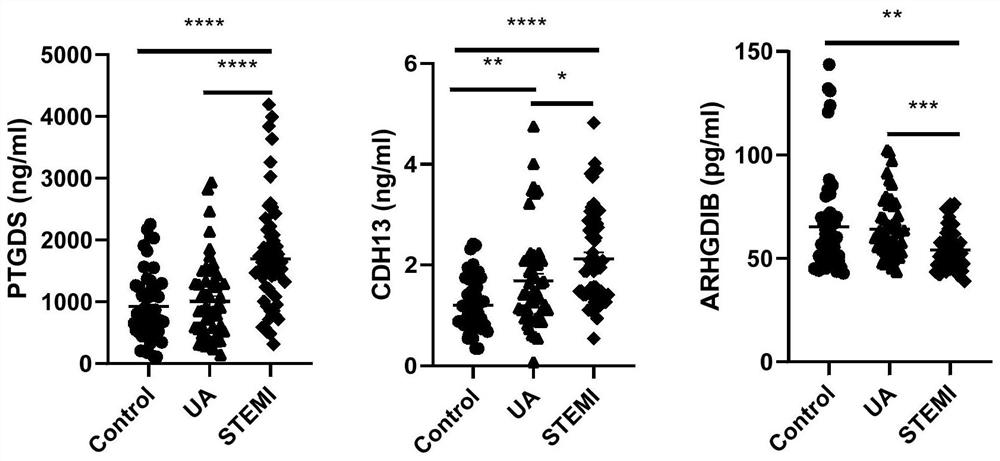
Protein composition, application thereof and kit for early screening and diagnosis of ST segment elevation type myocardial infarction
A technology of myocardial infarction and composition, applied in the field of molecular biology, can solve the problems of early diagnosis and low prognostic value, clinical sensitivity and specificity limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 1. Collection of Human Plasma Samples
[0098] ST-segment elevation myocardial infarction group and unstable angina pectoris group were patients with ST-segment elevation myocardial infarction and unstable angina pectoris in emergency department, and patients with negative coronary angiography were the control group. 2ml of whole blood was collected from each patient's peripheral vein, and immediately centrifuged at 2500rpm for 15 minutes to separate the upper layer of plasma. The separated plasma was then divided into several equal parts, placed in plasma collection tubes, and stored in a -80°C refrigerator for testing.
[0099] 2. Proteomic detection
[0100] 1) Plasma proteome detection
[0101] Data independent acquisition (DIA) technology was used to detect proteins in each mixed sample of the three groups.
[0102] 2) Protein extraction
[0103] a) Take 40 μl of plasma from each sample and dilute ten-fold with binding buffer (kit: Binding Buffer).
[0104] b)...
Embodiment 2
[0182] Enzyme-linked immunosorbent assay (ELISA)
[0183]Prostaglandin H2 D-isomerase, H-cadherin / H-cadherin, and Rho GDP dissociation inhibitor 2 were verified in freshly collected plasma samples by ELISA. Experimental principle: The antibody of the protein to be tested is pre-embedded at the bottom of the 96-well plate. After the standard and sample are added, the protein to be tested will bind to the antibody. After removal of unbound substrate, a biotin-conjugated antibody to the protein of interest is added. After washing, anti-biotin conjugated horseradish peroxidase-labeled antibody (HRP) was added, and unbound HRP was removed by washing. After adding a chromogenic reagent to terminate the reaction, measure the absorbance of the liquid.
[0184] Validated Prostaglandin H2 D-isomerase, Include:
[0185] Multi-well plate coated with prostaglandin H2 D-isomerase antibody
[0186] Pure prostaglandin H2 D-isomerase as a standard
[0187] Biotin-labeled antibody
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com