Related tolvaptan impurity, and synthesis method and application thereof
A synthesis method and compound technology, applied in the related impurities of tolvaptan, its synthesis field, can solve the problems such as no method to remove, difficulty in purity, etc., to achieve the effect of ensuring safety and effectiveness, simple method, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the influence of the amount of pyridine on the synthetic formula I compound
[0059]
[0060]Dissolve 15.0 g of 2-methyl-4-(2-methylbenzamido)benzoyl chloride (formula V) in 150 g of dichloromethane, and divide the solution into three parts: A, B and C: Add 1.38 g (1.0 equivalent) of pyridine, add 2.06 g (1.5 equivalent) of pyridine to B, and add 2.75 g (2.0 equivalent) of pyridine to C. React at room temperature (15-20°C) for 10 hours.
[0061] The reaction system was washed with 1 mol / L hydrochloric acid (50 mL) and saturated brine (20 mL), dried and concentrated over sodium sulfate, and the product was separated by column chromatography.
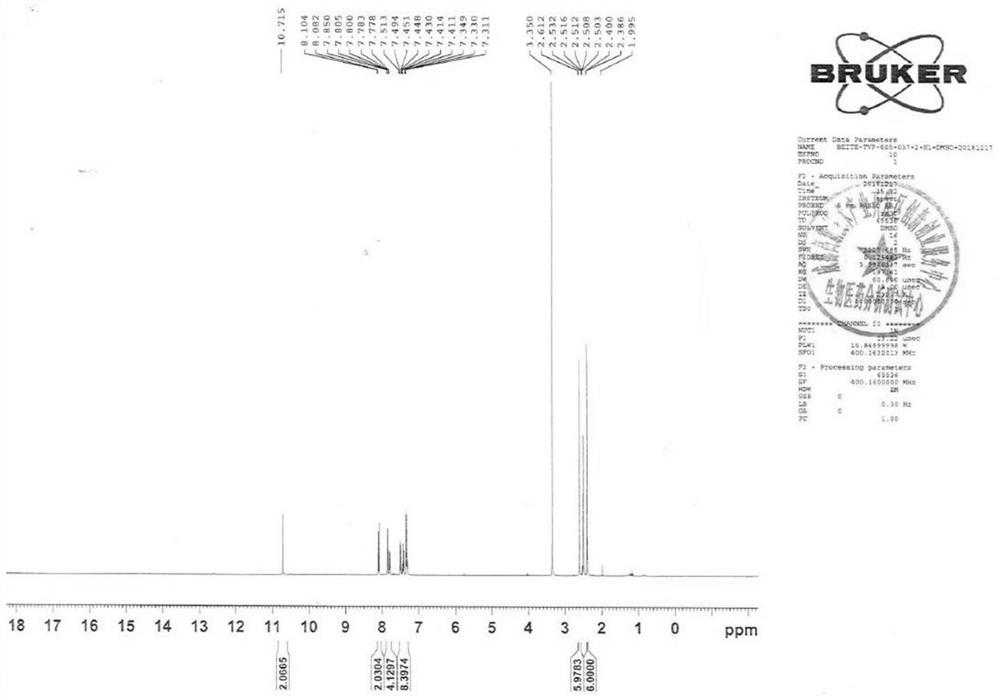
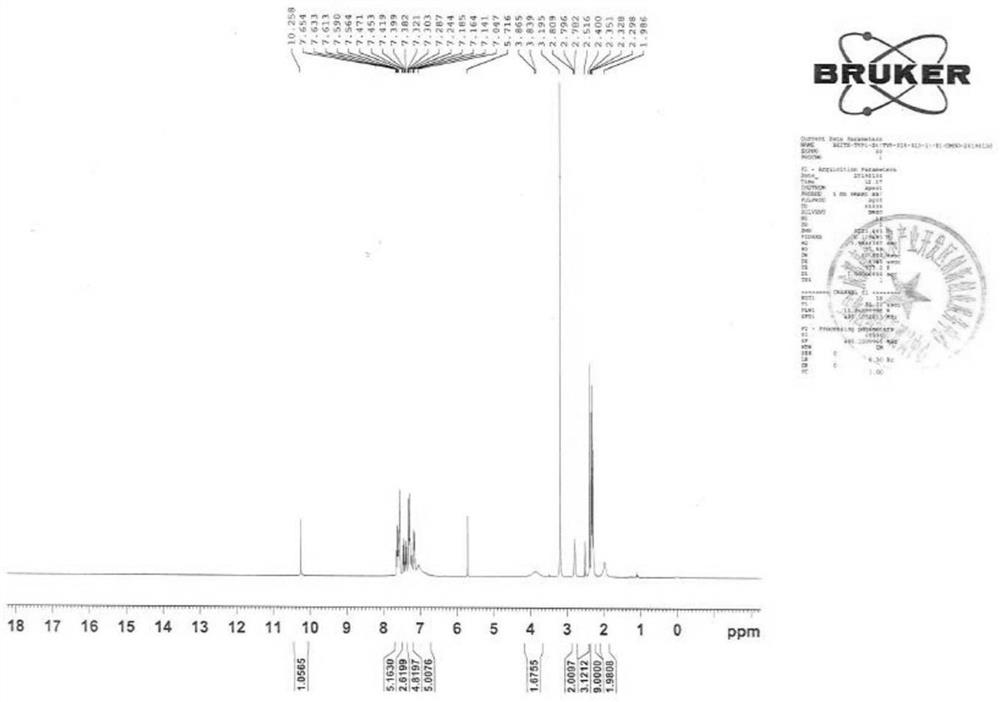
[0062] 1 H-NMR (400MHz, d 6 -DMSO): δ10.72(s,2H),8.09(d,J=8.8Hz,2H),7.85-7.78(m,4H),7.51-7.31(m,8H),2.80(s,2H), 2.61(s,6H),2.40(s,6H)ppm.
[0063] LC-MS: quasi-molecular ion 559.1621, [M+K] +
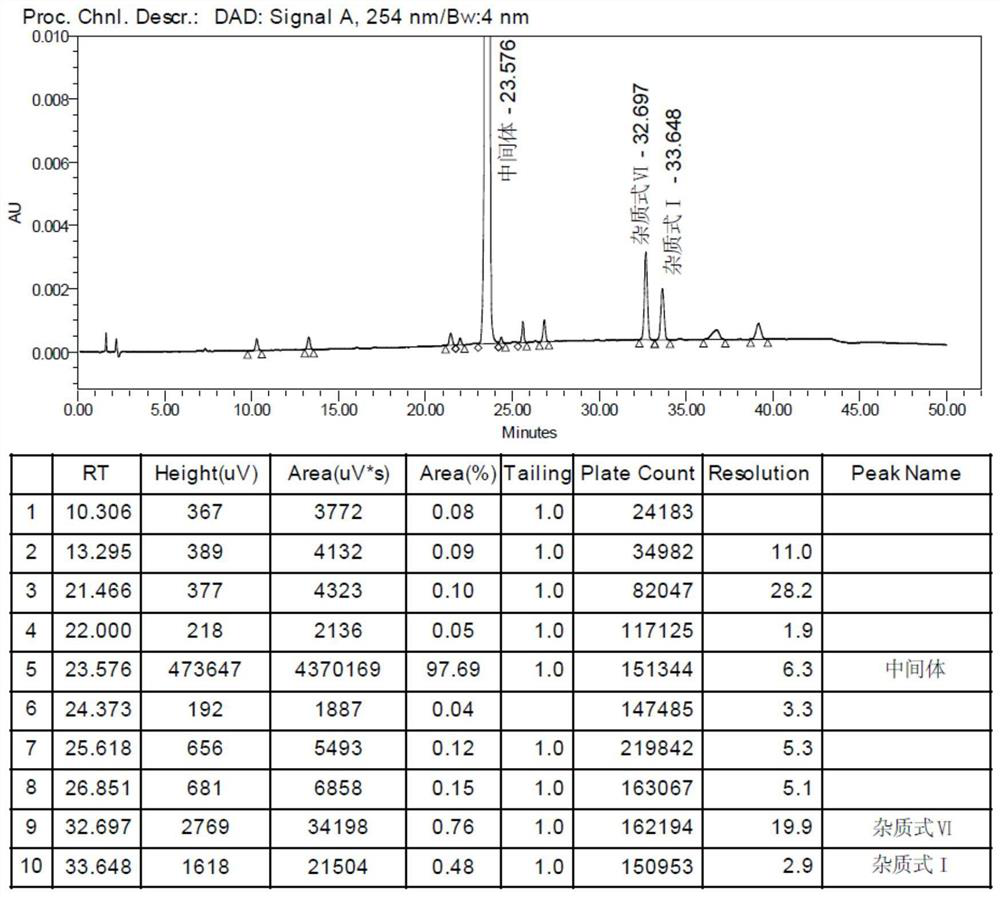
[0064] Each group of experimental formula I compound productive rate compares the following table:
[0065] Produc...
Embodiment 2
[0068] Embodiment 2: the influence of the amount of triethylamine on the synthetic formula I compound
[0069] Add 185.6 g of dichloromethane to 18.2 g of 2-methyl-4-(2-methylbenzamido)benzoyl chloride (Formula V), and dissolve the solution in three parts: A, B and C: A 2.14 g (1.0 equivalent) of triethylamine was added to B, 3.22 g (1.5 equivalent) of triethylamine was added to B, and 4.28 g (2.0 equivalent) of triethylamine was added to C. React at room temperature (20-25°C) for 8 hours.
[0070] The reaction system was washed with 1 mol / L hydrochloric acid (50 mL) and saturated brine (20 mL), dried and concentrated over sodium sulfate, and the product was separated by column chromatography.
[0071] Each group of experimental formula I compound productive rate compares the following table:
[0072] Table 2 different triethylamine consumption yield contrast
[0073] Experimental group Triethylamine equivalents Formula I Yield molar yield A 1.0 2.36g ...
Embodiment 3
[0075] Embodiment 3: the influence of the amount of N-methylmorpholine on the synthetic formula I compound
[0076] Add 220.6 g of dichloromethane to 21.3 g of 2-methyl-4-(2-methylbenzamido)benzoyl chloride (Formula V), and dissolve the solution, and divide the solution into three parts A, B and C: A 2.51 g (1.0 equivalent) of N-methylmorpholine was added to B, 3.80 g (1.5 equivalent) of N-methylmorpholine was added to B, and 5.02 g (2.0 equivalent) of N-methylmorpholine was added to C. React at room temperature (20-25°C) for 8 hours.
[0077] The reaction system was washed with 1mol / L hydrochloric acid (50ml) and saturated brine (20ml) respectively, dried over sodium sulfate, and the product was separated by column chromatography.
[0078] Each group of experimental formula I compound productive rate compares the following table:
[0079] Table 3 different N-methylmorpholine dosage yield contrast
[0080] Experimental group N-methylmorpholine equivalents Form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com