Naphthalimide compound as well as preparation method and application thereof
A naphthalimide and compound technology, applied in the field of photochromic materials, can solve the problems of complex synthesis, fast light response speed, low total yield and the like, and achieve the effects of simple synthesis and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment prepares a kind of naphthalimide compound shown in the following structure:
[0050]
[0051] The preparation method is as follows:
[0052]
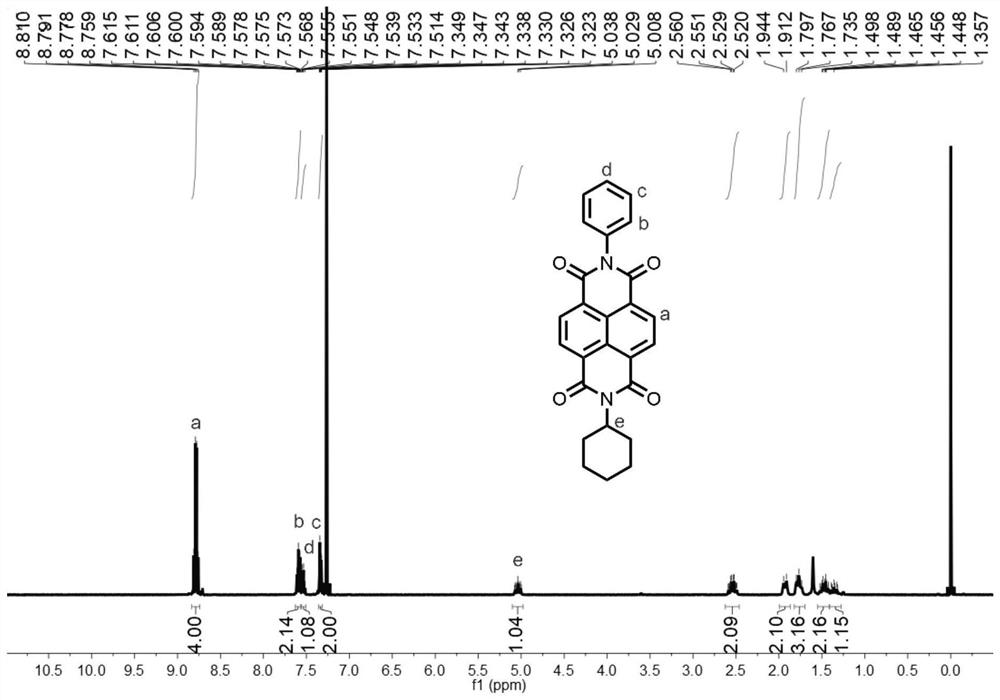
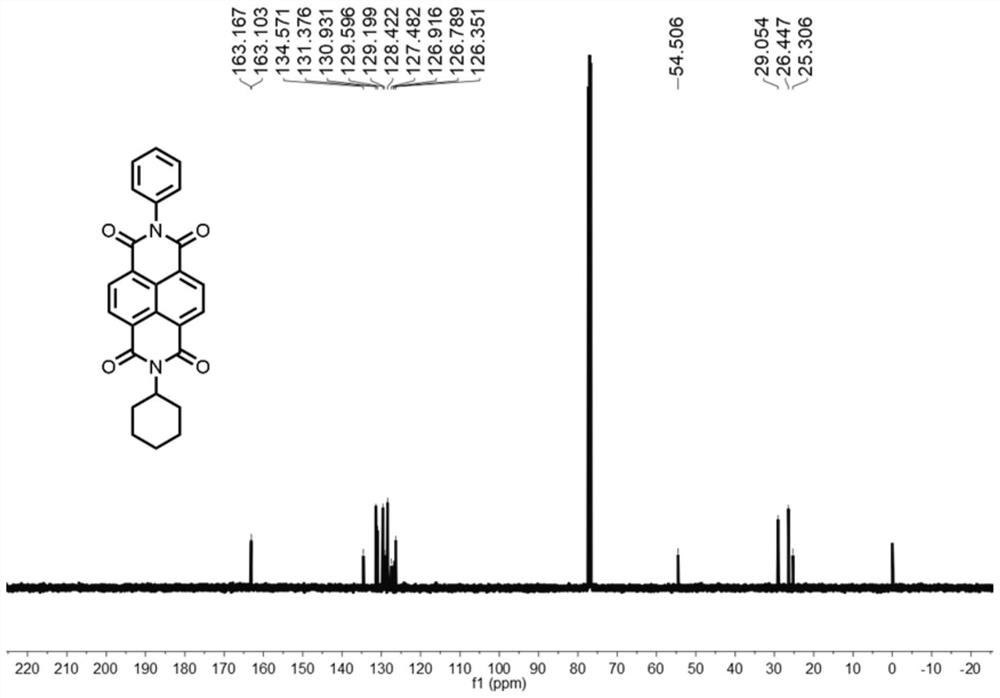
[0053]1,4,5,8-Naphthalenetetracarboxylic anhydride (1.41 g, 5.26 mmol) was dissolved in 50 mL of N,N-dimethylformamide, cyclohexylamine (0.52 g, 5.26 mmol)) and aniline (0.49 g, 5.26 mmol), heated to 150 °C for 24 h; the reaction solution was cooled to 20 °C, poured into 500 mL of water, filtered, and the filter cake was subjected to column chromatography (the stationary phase was silica gel, and dichloromethane was the mobile phase) to obtain the Naphthalimide compounds.
[0054] The hydrogen nuclear magnetic spectrum and carbon spectrum of the naphthalimide compounds are as follows figure 1 and figure 2 As shown, the specific chemical shifts are: 1 H NMR (400MHz, CDCl 3 )δ=8.82-8.75(m, 4H), 7.62-7.56(m, 2H), 7.56-7.50(m, 1H), 7.36-7.31(m, 2H), 5.04(tt, J=12.2, 3.7Hz, 1H), 2.54(qd, J=12.4, 3.5Hz, 2H)...
Embodiment 2
[0057] The present embodiment prepares a naphthalimide compound shown in the following structure:
[0058]
[0059] The preparation method is as follows:
[0060]
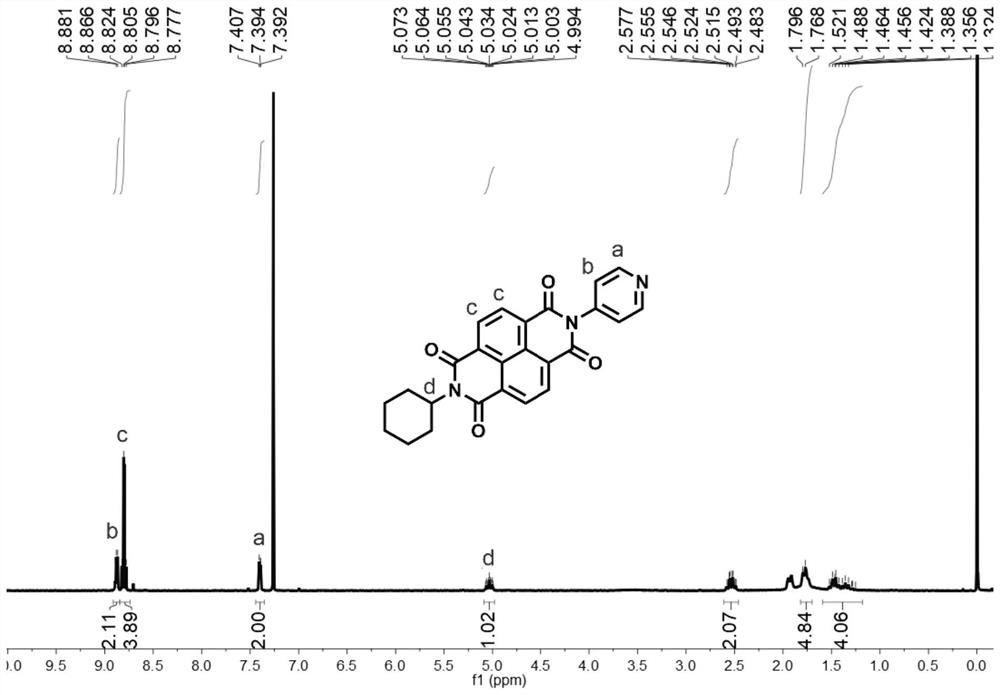
[0061] 1,4,5,8-Naphthalenetetracarboxylic anhydride (1.41g, 5.26mmol) was dissolved in 50mL N,N-dimethylformamide, cyclohexylamine (0.52g, 5.26mmol)) and 4-amino were added Pyridine (0.50 g, 5.26 mmol) was heated to 130 °C for 36 h; the reaction solution was cooled to 20 °C, poured into 500 mL of water, filtered, and the filter cake was passed through column chromatography (the stationary phase was silica gel, and chloroform was the mobile phase), The naphthalimide compound is obtained.
[0062] The hydrogen nuclear magnetic spectrum and carbon spectrum of the naphthalimide compounds are as follows image 3 and Figure 4 As shown, the specific chemical shifts are: 1 H NMR (400MHz, CDCl 3 )δ=8.87(d,J=6.3Hz,2H),8.83-8.76(m,4H),7.40(d,J=5.8Hz,2H),5.03(tt,J=12.3,3.7Hz,1H), 2.63-2.41 (m, 2H), 1.87-1.70 (m, 4...
Embodiment 3
[0065] The present embodiment prepares a naphthalimide compound shown in the following structure:
[0066]
[0067] The preparation method is as follows:
[0068]
[0069] 1,4,5,8-Naphthalenetetracarboxylic anhydride (1.41 g, 5.26 mmol) was dissolved in 50 mL of ethanol, cyclohexylamine (0.52 g, 5.26 mmol)) and 3-aminopyridine (0.50 g, 5.26 mmol) were added , heated to 110°C for 48h; the reaction solution was cooled to 20°C, poured into 500 mL of water, filtered, and the filter cake was passed through column chromatography (the stationary phase was silica gel and chloroform was the mobile phase) to obtain the naphthalimides compound.
[0070] The hydrogen nuclear magnetic spectrum and carbon spectrum of the naphthalimide compounds are as follows Figure 5 and Image 6 As shown, the specific chemical shifts are: 1 H NMR (400MHz, DMSO-d 6 )δ8.84-8.53(m,7H),7.94(dt,J=8.1,1.7Hz,2H),7.64(dd,J=8.0,4.8Hz,1H),5.00-4.79(m,1H),2.47 -2.35(m, 2H), 1.92-1.74(m, 3H), 1.71(d, J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com