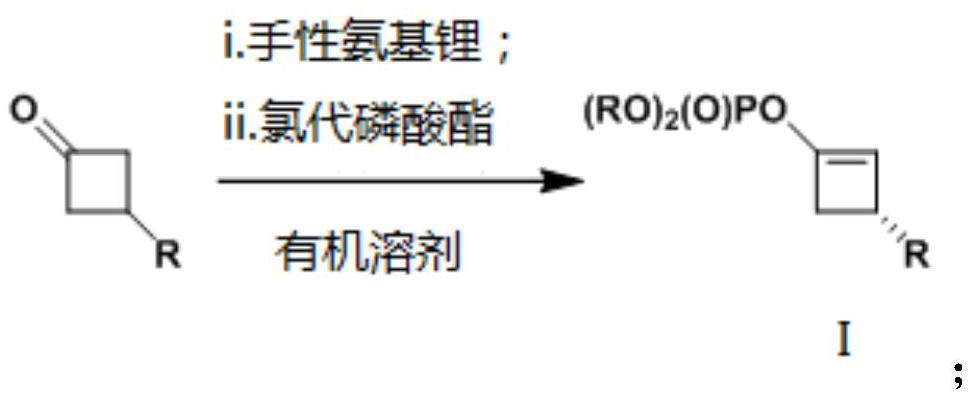
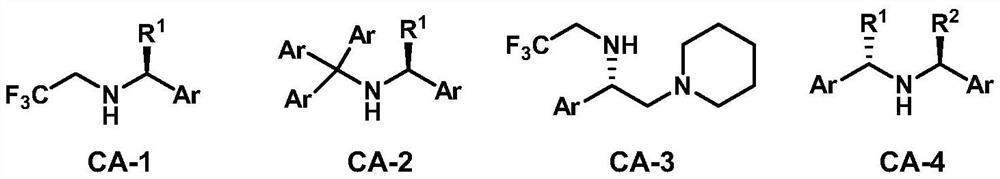
Method for desymmetrization of 3-substituted cyclobutanone, cyclobutene compound and use thereof
A cyclobutanone and desymmetry technology, applied in the field of chemical synthesis, can solve the problems of little research, difficult direct conversion of 3-substituted cyclobutanone, poor stability, etc., and achieves simple operation, wide substrate universality, High stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] To a dry Schlenk reaction tube was added the chiral amine hydrochloride CA-4c·HCl (0.127 g, 0.440 mmol), THF (1.2 mL) sequentially. After cooling to -78 °C, n-butyllithium (2.28 M, 0.39 mL, 0.880 mmol) was added dropwise. After stirring for five minutes, the cooling bath was removed, and after returning to room temperature, stirring was continued for 15 minutes until the reaction system became clear. The reaction tube was then placed in a -90°C cold bath, and a solution of 3-substituted cyclobutanone 1a (58.5 mg, 0.400 mmol) in tetrahydrofuran (0.5 mL, 0.3 mL wash, 0.2 mL) was added dropwise over a period of 30 minutes washing). After the addition was completed, the reaction was held for 30 minutes, and the electrophile ClP(O)(OPh) was added dropwise over a period of 10 minutes. 2 (0.42 mL, 0.540 g, 2.00 mmol), after holding the reaction for 30 minutes, the temperature of the cooling bath was increased to -78°C. After continuing the reaction for about 3.5...
Embodiment 2
[0039]
[0040] The operation is the same as in Example 1. The raw materials and dosage are as follows: 3-substituted cyclobutanone 1b (64.0 mg, 0.400 mmol), chiral amine hydrochloride CA-4c HCl (0.127 g, 0.440 mmol), n-butyllithium (2.25 M, 0.39 mL) , 0.880mmol) and the electrophile ClP(O)(OPh) 2 (0.42 mL, 0.540 g, 2.00 mmol). Flash column chromatography (eluent: petroleum ether / ethyl acetate = 10 / 1) to obtain chiral cyclobutene phosphate 2b (0.155 g, 85%): colorless oil; 93% ee (HPLC condition: OD -H column, cyclohexane / i-PrOH=95 / 5, 1.0mL / min, λ=210nm, t R (main)=10.5min,t R (minor) = 11.8min); (c 0.50, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ(ppm): 7.41-7.32(m, 4H), 7.30-7.19(m, 7H), 7.15-7.02(m, 3H), 5.40(s, 1H), 3.64(d, J=3.4Hz, 1H ), 3.27(ddd, J=13.2, 4.5, 1.5Hz, 1H), 2.57(d, J=13.2Hz, 1H), 2.32(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ(ppm): 150.29(d, J=7.3Hz), 141.98(d, J=9.9Hz), 139.04, 136.19, 129.89, 129.06, 126.46, 125.68, 120.05(d, J=4.9Hz), 120.04( d, J=5.0H...
Embodiment 3
[0042]
[0043] The operation is the same as in Example 1. The raw materials and dosage are as follows: 3-substituted cyclobutanone 1c (47.4 mg, 0.272 mmol), chiral amine hydrochloride CA-4c HCl (96.0 g, 0.330 mmol), n-butyllithium (2.25 M, 0.29 mL) , 0.660 mmol) and the electrophile ClP(O)(OPh) 2 (0.31 mL, 0.400 g, 1.50 mmol). Flash column chromatography (eluent: petroleum ether / ethyl acetate = 95 / 5) to obtain chiral cyclobutene phosphate 2c (97.4 mg, 88%): colorless oil; 93% ee (HPLC condition: OJ -H column, cyclohexane / i-PrOH=9 / 1, 1.0mL / min, λ=210nm, t R (main)=7.0min,t R (minor) = 8.2min); (c 1.0, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ(ppm): 7.45-7.30(m, 4H), 7.30-7.17(m, 6H), 6.85(s, 3H), 5.41(s, 1H), 3.63-3.59(m, 1H), 3.26(ddd , J=13.2, 4.5, 1.5Hz, 1H), 2.59 (d, J=13.2Hz, 1H), 2.28 (s, 6H); 13 C NMR (101MHz, CDCl 3 )δ(ppm): 150.27(d, J=7.3Hz), 142.00, 141.87(d, J=9.9Hz), 137.91, 129.87, 128.27, 125.67, 124.34, 120.02(d, J=4.9Hz), 120.01( d, J=4.9Hz), 113.89 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com