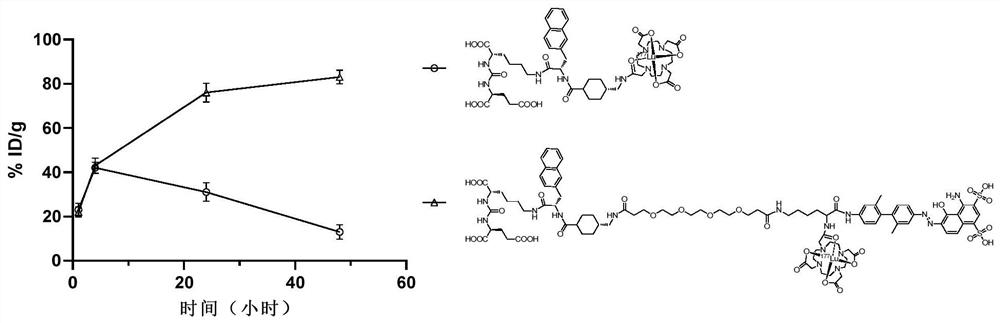
Prostate-specific membrane antigen targeting compound with long circulation half-life period as well as preparation method and application thereof
A prostate-specific compound technology, applied in the field of nuclear medicine and molecular imaging, can solve the problems of insufficient combination of drugs and targets, inability to meet therapeutic purposes, low effective dose, etc., to enhance tumor uptake enrichment and retention time, prolong The effect of blood circulation half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of formula (II-1) compound 10
[0069] Synthesis of Compound 2:
[0070] 4,4'-diamino-3,3'-dimethylbiphenyl (compound 11) (2.12g, 10.0mmol), di-tert-butyl dicarbonate (2.2g, 10.0mmol) and N,N-Diisopropylethylamine (1.3g, 10.0mmol) and 20mL of dichloromethane were stirred overnight at room temperature, and the completion of the reaction was monitored by HPLC (r.t. was 10.13 minutes). Column purification (petroleum ether / ethyl acetate=5:1) yielded compound 2 as a white solid with a yield of 59%.
[0071] Synthesis of compound 3:
[0072] In a 50mL flask, compound 2 (0.31g, 1.0mmol) and 4mL of acetonitrile were dropped into the reaction flask in an ice bath, and 1.5mL of 2M hydrochloric acid was added dropwise to the reaction flask, reacted for 15min, and sodium nitrite (0.068g, 1.0mmol) was added ) was dissolved in 2mL of water, added dropwise into the reaction bottle again, reacted for half an hour, and used as A liquid for later use. In...
Embodiment 2-6
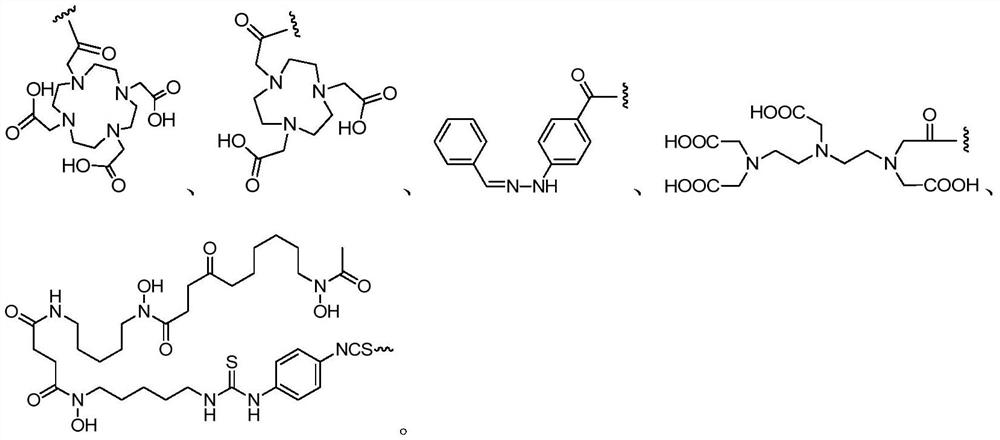
[0090] The compound structure of embodiment 2-6 is as shown in formula (II-2) to formula (II-6), and their preparation method all can refer to embodiment 1, in formula (II-2) and formula (II-3) Preparation of COOH-PEG that will react with compound 6 4 -COOH is replaced by COOH-PEG 2 -COOH, malonic acid or other suitable compounds; in the preparation of formula (II-4) to formula (II-6), the Nα-Fmoc-Nε-Boc-L-lysine of compound 4 reaction is replaced by Boc glycine , while replacing PSMA-617 reacted with compound 7 with PSMA-617-(Fmoc)Lys-, the following corresponding structure is obtained:
[0091]
[0092] or
[0093]
Embodiment 7-30
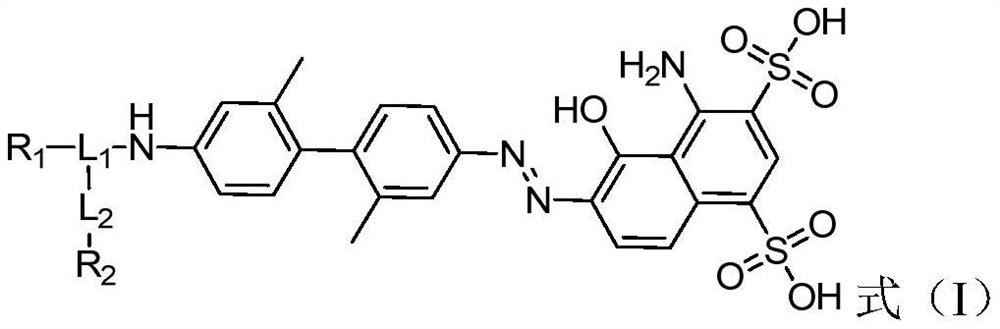
[0095] With reference to the preparation method of Example 1-6, prepare the compound expressed by the following formula (I):
[0096]
[0097]
[0098]
[0099]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com