Method for detecting nitrosamine compounds in higenamine hydrochloride
A technology for higenamine hydrochloride and nitrosamines, which is applied in the field of chemical detection and analysis, and can solve the problems of introducing nitrosamine impurities, nitrosamine impurities, and no detection method for nitrosamine impurities is reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
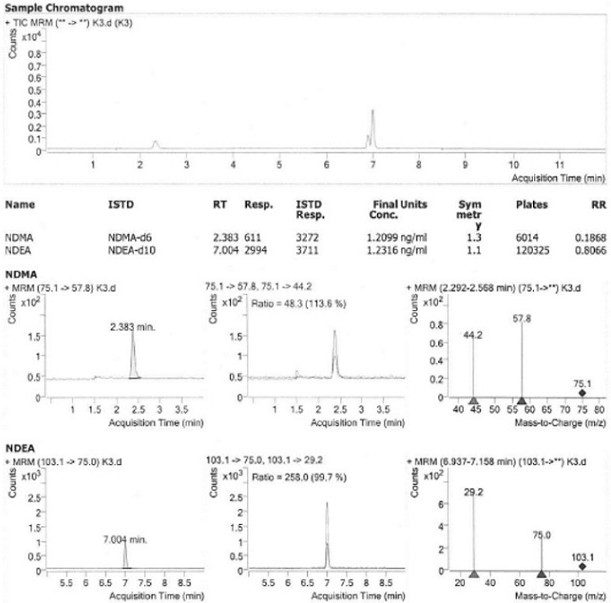
[0031] Instrument and Reagent Information
[0032] Liquid Chromatography-Mass Spectrometry-Agilent; Electronic Analytical Balance-METTLER TOLEDO; Chromatographic Column-Agilent Waters HSS-T3 100 x 3.0mm, 1.8μm; Methanol-Merck HPLC; Formic Acid-Tianjin Kemiou Chemical Reagent Co., Ltd- HPLC; Ultrapure Water-Zhuhai Rundu Pharmaceutical Co., Ltd.-HPLC; N-Nitrosodimethylamine (NDMA)-TCI; N-Nitrosodiethylamine (NDEA)-Bailingwei Technology Co., Ltd.; NDMA-d6 CATO; NDEA-d10 CATO; higenamine hydrochloride-Zhuhai Rundu Pharmaceutical Co., Ltd.
[0033] 2) Preparation of solution
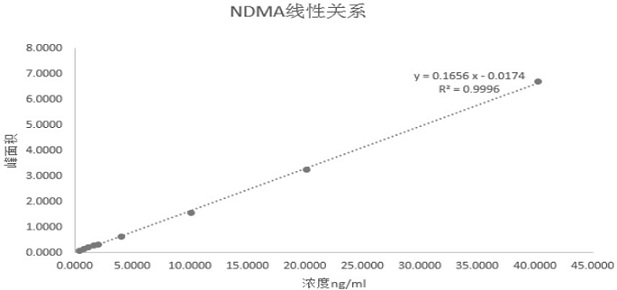
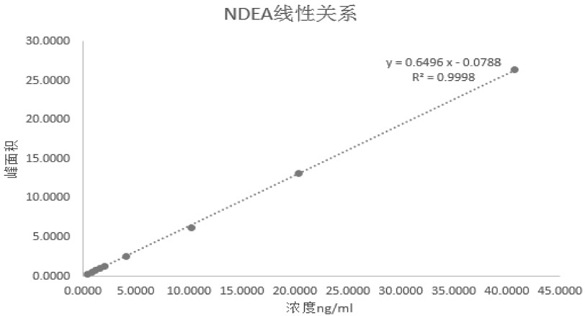
[0034] (1) Standard curve solution: use methanol, NDMA and NDEA to prepare the reference solution, where C NDMA =40ng / ml, C NDEA =40 ng / ml, using water and methanol as diluents, respectively, 0.4, 0.8, 1.2, 1.6, 2, 4, 10, 20, 40 ng / ml (marked as K1, K2, K3, K4, K5, K6, K7, K8, K9) series of NDMA standard curve solutions, respectively 0.4, 0.8, 1.2, 1.6, 2, 4, 10, 20, 40 ng / ml (marked as K1, K2, K3, K4, K5...
Embodiment 2
[0043] Embodiment 2 carries out analytical method verification to the method that the present invention develops, and the result obtained is shown in the table below:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com