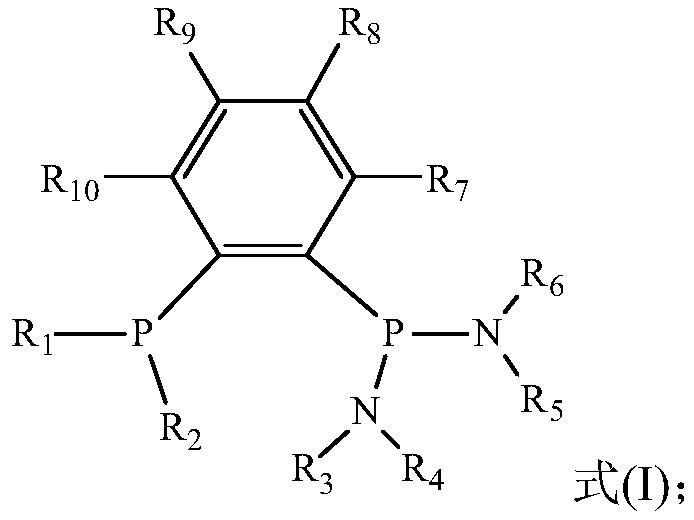
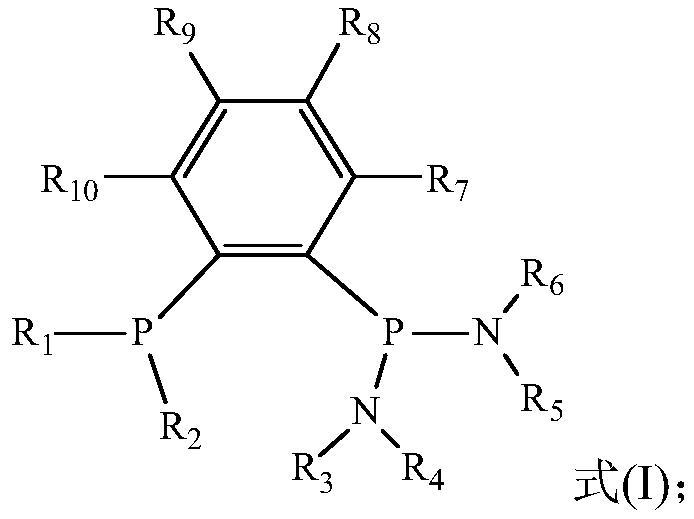
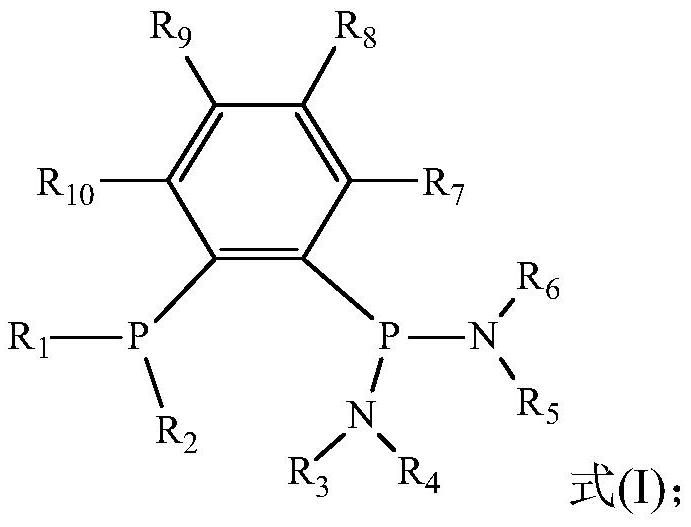
Catalyst system for ethylene selective oligomerization, reaction method and application of reaction method
A catalyst and selectivity technology, applied in catalytic reactions, catalysts, carbon compound catalysts, etc., can solve the problems of low total selectivity of α-olefins and high methylenecyclopentane content, and achieve high selectivity and total selectivity. High, high catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] In an embodiment of the present invention, the preparation method of ligand a may include the following steps:
[0052] (1) Preparation of Ph 2 PPhBr
[0053] Take a certain amount of 1,2-dibromobenzene, add a small amount of n-hexane and cool it in the refrigerator for 10-20 minutes for later use. Take a certain amount of n-BuLi, add a small amount of n-hexane and put it in the refrigerator to cool for 10-20 minutes, then take out the above two medicines, slowly add n-BuLi to the above standby solution, stir and react for about 30 minutes, add a small amount of n-hexane Ph after alkane dilution 2 PCl was added dropwise and stirred overnight. After the reaction is complete, use a sand core funnel to filter out the lithium salt, and the solution is distilled under reduced pressure to remove the solvent to obtain the colorless oily product Ph 2 PPhBr.
[0054] (2) Preparation of Ph 2 PPhP (NR 3 R 4 )(NR 5 R 6 )
[0055] Take a certain amount of Ph 2 Add approp...
Embodiment 1
[0067] 1. Preparation of N,N,N',N'-tetramethyl-1-(2-(methyl(phenyl)phosphino)phenyl)-phosphinediamine (L1):
[0068]
[0069] Take (2-bromophenyl)(methyl)(phenyl)phosphine (1.71g, 5mmol) and add an appropriate amount of n-hexane and put it in the refrigerator for later use. Take a certain amount of n-BuLi (0.32g, 5mmol), add a small amount of n-hexane Put the alkanes in the refrigerator to cool for 10-20 minutes, then take out the above two drugs, slowly add n-BuLi dropwise to the above standby solution, and stir for about 30 minutes. Take 1-chloro-N,N,N',N'-tetramethylphosphinediamine (0.77g, 5mmol) and add an appropriate amount of n-hexane into the refrigerator. After 15-20min, take out the above solution and put 1-chloro-N ,N,N',N'-Tetramethylphosphinediamine was slowly added to the above mixed solution, stirred overnight, then filtered and vacuum-dried to obtain a yellow oil, then added an appropriate amount of n-hexane and stirred, mixed thoroughly and put into the ref...
Embodiment 2
[0076] With embodiment 1. The difference is that R 3 , R 4 is ethyl, R 5 , R 6 For ethyl. The distribution of oligomerization products is shown in Table 1, and the experimental conditions and catalyst activity are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com