Synthesis method of telmisartan intermediate
A synthetic method, telmisartan technology, applied in the field of synthesis of telmisartan intermediates, can solve the problems of high reaction temperature, high equipment requirements, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
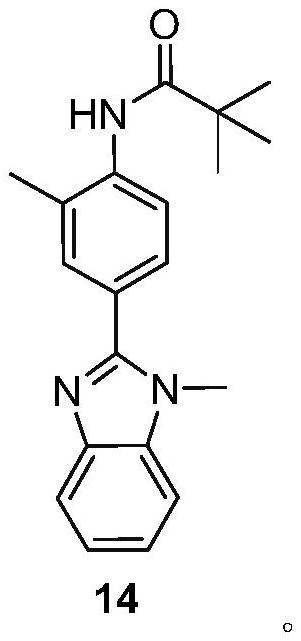
[0037] Preparation of Compound (14)
[0038] Take a 500mL three-necked flask, add 53.92g (0.27mol) of compound (13) and 150mL of methanol in sequence, stir to dissolve, add 50g (0.23mol) of compound (12), reflux for 5h, add saturated sodium bisulfite solution, and then reflux for 1h, TLC detects that the raw material point disappears, and the heating is stopped. Suction filtration, washing with 30 mL of methanol, the filtrate was dried under reduced pressure, and the concentrate was recrystallized with 50% aqueous ethanol to obtain 67.37 g of off-white solid as compound (14), melting point 175.4-176.1°C, yield 92%.
[0039] 1 H NMR (400MHz, DMSO-d 6 )δ9.00(brs,1H),7.73(d,J=1.2Hz,1H),7.67–7.64(m,2H),7.61(d,J=7.8Hz,1H),7.42(d,J=8.2 Hz,1H),7.27(m,2H),3.89(s,3H),2.28(s,3H),1.27(s,9H).
[0040] 13 C NMR (150MHz, CDCl 3 )δ176.75, 153.58, 143.00, 137.53, 136.72, 131.76, 128.61, 127.73, 126.21, 122.78, 122.50, 122.07, 119.77, 109.69, 40.07, 31.84, 27.81, 17.69.
[0041] MS-ESI:...
Embodiment 2
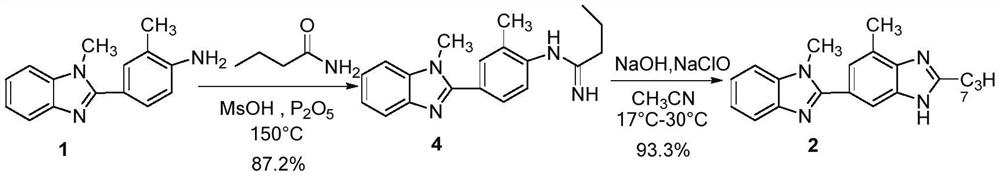
[0043] Preparation of compound (1)
[0044] Take the microwave reaction flask, add 48.2g (0.15mol) compound (14), 24.3g (0.60mol) sodium hydroxide, 140mL 95% ethanol in sequence, reflux reaction for 5h, point the plate to monitor the reaction of raw materials, stop the reaction, and spin down under reduced pressure Dry to get the crude product. Recrystallized with 35% ethanol aqueous solution to obtain 33.8 g off-white solid powder as compound (1), with a measured melting point of 147.4-149.9°C and a yield of 95%.
[0045] 1 H NMR (400MHz, DMSO-d 6)δ7.59–7.57(m,1H),7.53–7.51(m,1H),7.46(s,1H),7.41(dd,J=8.4,1.6Hz,1H),7.24-7.15(m,2H) ,6.73(d,J=8.4Hz,1H),5.36(brs,2H),3.84(s,3H),2.14(s,3H).
[0046] MS-ESI: m / z 238.1[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com