Pharmaceutical composition for alleviating eye fatigue, containing, as active ingredients, luteolin-7-o-diglucuronide and apigenin-7-o-diglucuronide isolated from perilla frutescens (l.) britton var. acuta (thunb.) kudo leaf extract
A technology of glucuronide and perilla leaf extract, which is applied in the direction of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., can solve the problems of decreased dark adaptation ability and easy fatigue of the eyes, and achieve improved eye health. The effect of fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
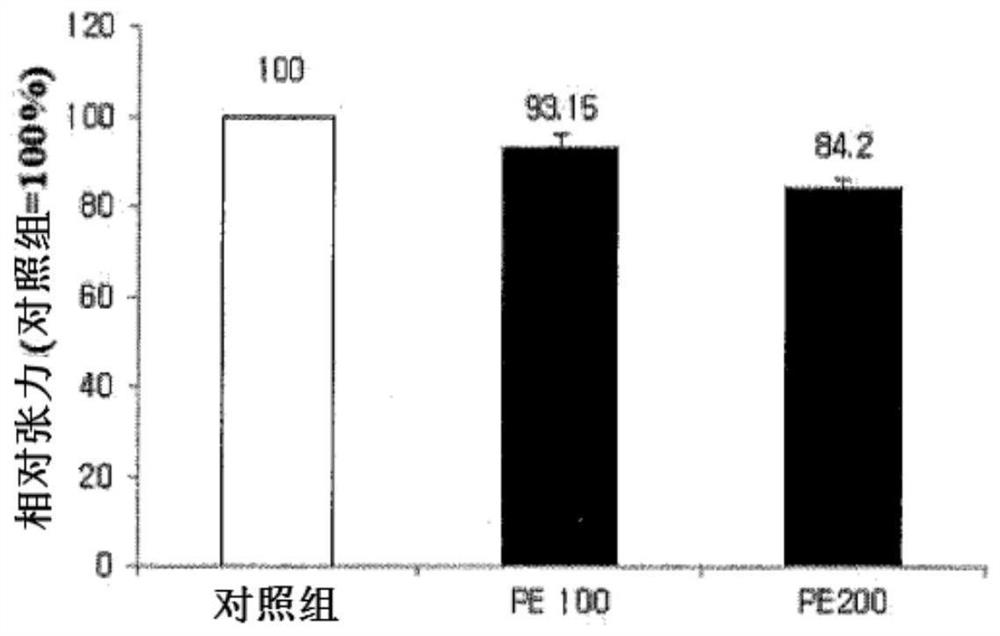
[0035] Embodiment 1. The effect of perilla extract in aortic smooth muscle cells (in vitro) and rabbit ciliary muscle (in vitro), adjusting proximal point (human body applicable test)
[0036] 1. Preparation of hot water extraction of perilla leaves
[0037] 3 kg of perilla leaves (dried leaves) were extracted with hot water at 100° C. for 3 hours using 10 times as much distilled water. The water extract extracted above was concentrated under reduced pressure and freeze-dried to obtain 650 g of hot water freeze-dried perilla leaves.
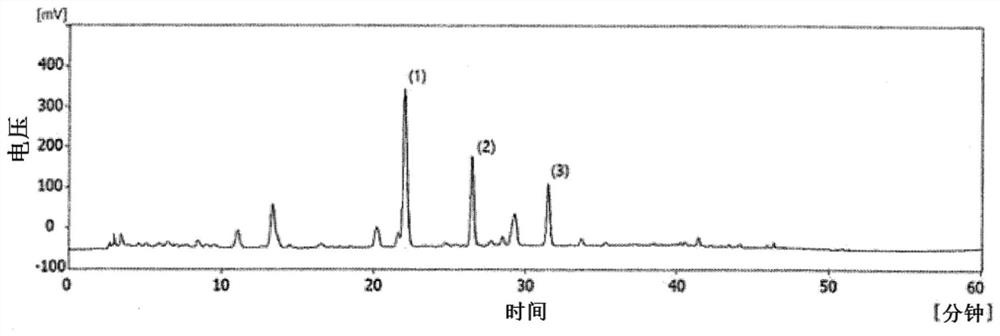
[0038] 2. Use HPLC to analyze the components of perilla extract
[0039] The HPLC device used in the component analysis of perilla leaf hot water extract is YL 9100HPLC system, and the chromatographic column uses Triart C18 plus (250x 4.6mm, 5um, YNC Co., Ltd.). Mobile phase is methanol (mobile phase A) and distilled water for HPLC (mobile phase B, 0.1% formic acid), and the ratio of methanol is adjusted from 30% (0-10 minutes) to 30% ~ 50% (10...
Embodiment 2
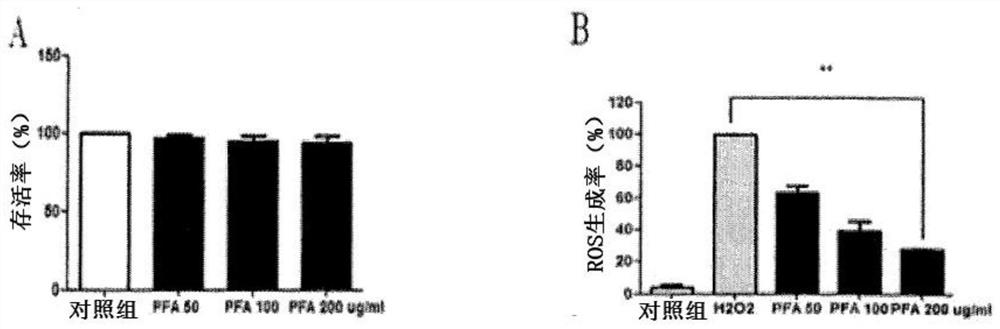
[0060] Example 2. Effects of luteolin-7-O-diglucuronide and apigenin-7-O-diglucuronide contained in perilla leaf extract on ciliary muscle isolated from SD rats Cellular Eyestrain Effects
[0061] 1. Preparation of perilla leaf hot water extract and isolation of flavonoid glycoside compounds
[0062] 1-1. Preparation of perilla leaf water extract
[0063] The dried perilla leaves were subjected to hot water extraction at 100° C. for 3 hours with distilled water. The above extract was concentrated under reduced pressure and then freeze-dried and spray-dried to obtain hot water freeze-dried perilla leaves (650 g, 21.6%) and spray-dried (669 g, 22.4%).
[0064] 1-2. Separation of active ingredients using formaldehyde-based resin HP-20 (Diaion HP-20resin)
[0065] In order to separate the effective components from perilla leaf extract, 2L of perilla leaf hot water extract was added to formaldehyde-based resin HP-20, and water and methanol were sequentially mixed at 30:70, 50:50...
Embodiment 3
[0100] Embodiment 3: the human body application test of eye regulation
[0101] 1. Applicable test method for human body
[0102] This study was conducted under the approval of the Medical Device Clinical Trials Review Board of D University Hospital (IRB license number: DSGOH-033).
[0103] A total of 35 adult men and women aged 18+ to 60+ were screened, and the subjects voluntarily gave their written consent after receiving an explanatory note for the study. No congenital or chronic diseases; results of medical diagnosis and treatment, no pathological symptoms or characteristics; results of blood tests and vital signs within the normal range; A total of 30 people were finally studied. The study was conducted by taking perilla extract and placebo control group, and performing close proximity task (VDT) for 2 hours before the final dose.
[0104] 2. Adjust near point (Near Point of Accomodation) check
[0105] Adjusting the near point is measured as follows: In the state of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com