Preparation method of tablet with improved performance
A technology for improving performance and tablet, applied in the field of pharmaceutical preparations to prevent sticking and punching problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
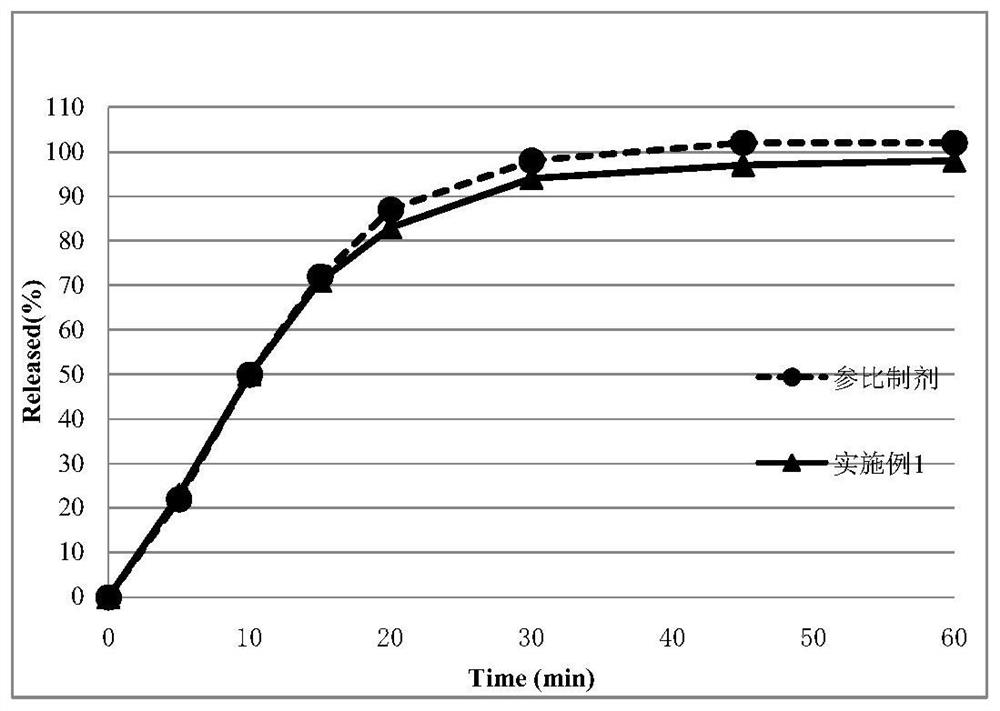
Embodiment 1
[0091] Adopt the prescription of following table 3, prepare tablet according to the preparation method of the present invention.
[0092] Table 3: tablet prescription of the present invention
[0093]
[0094] (1) The materials clopidogrel hydrogen sulfate, mannitol M100, polyethylene glycol 6000 and low-substituted hydroxypropyl cellulose LH-21 are passed through a 1.0mm sieve with a pulverizer; the sieved materials Place in a mixing hopper, set the rotation speed at 10 rpm, and mix for 15 minutes to obtain a mixed material.
[0095] (2) Place the above mixed material in the feed bin of the fluidized bed. Set the temperature of the material at 65°C, start the fluidized bed, and start granulation. When the material is observed to be wet without dry powder, the granules have been formed, and the granulation end point has been reached. Turn off the heating, lower the temperature of the granules to below 30°C, take out the granules, and pass through a 1.0mm sieve with a gran...
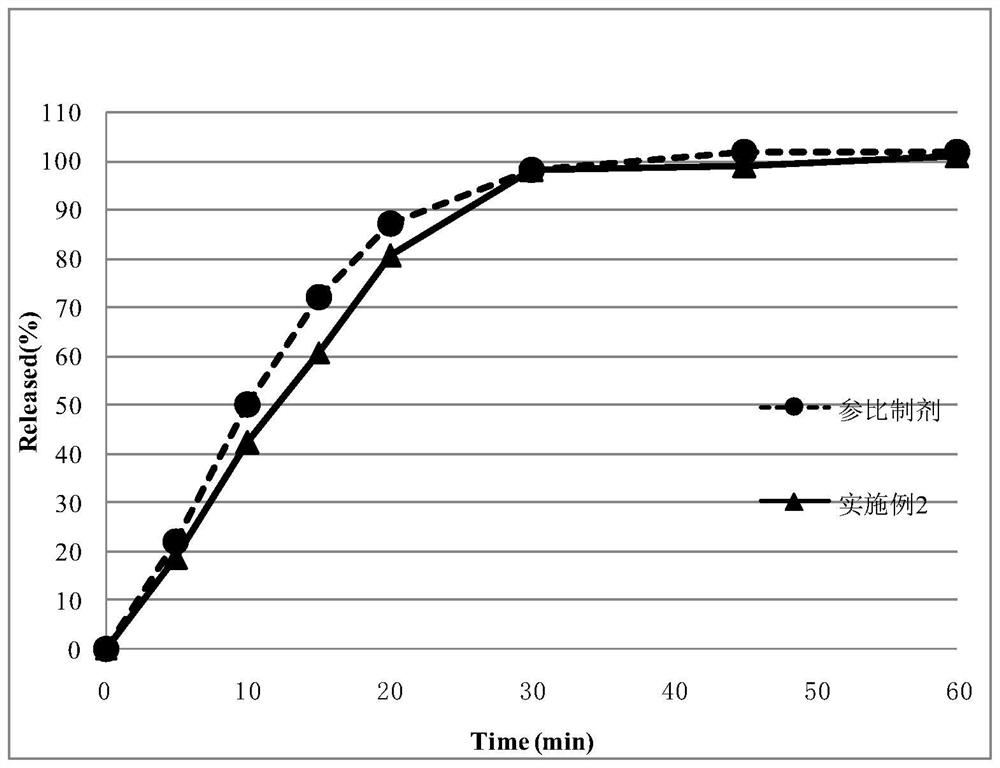
Embodiment 2
[0108] Tablets were prepared according to the preparation method of the present invention by adopting the prescription in the following Table 5.
[0109] Table 5: tablet prescription of the present invention
[0110]
[0111] (1) Pass the materials clopidogrel hydrogen sulfate, sorbitol CP40 / 200, polyethylene glycol 6000, and low-substituted hydroxypropyl cellulose LH-21 through a 1.0mm sieve with a pulverizer; The material is placed in the mixing hopper and the rotating speed is set at 10 rpm, and mixed for 15 minutes to obtain the mixed material.
[0112](2) Place the above mixed material in the feed bin of the fluidized bed. Set the temperature of the material to 60°C, start the fluidized bed, and start granulation. When the material is observed to be wet without dry powder, the granules have been formed, and the end point of granulation is reached. Turn off the heating, lower the temperature of the granules to below 30°C, take out the granules, and pass through a 1.0m...
Embodiment 3
[0131] Tablets were prepared according to the preparation method of the present invention by adopting the prescription in the following Table 7.
[0132] Table 7: tablet prescription of the present invention
[0133]
[0134] Tablets were prepared by the same process as in Example 1.
[0135] Results and discussion:
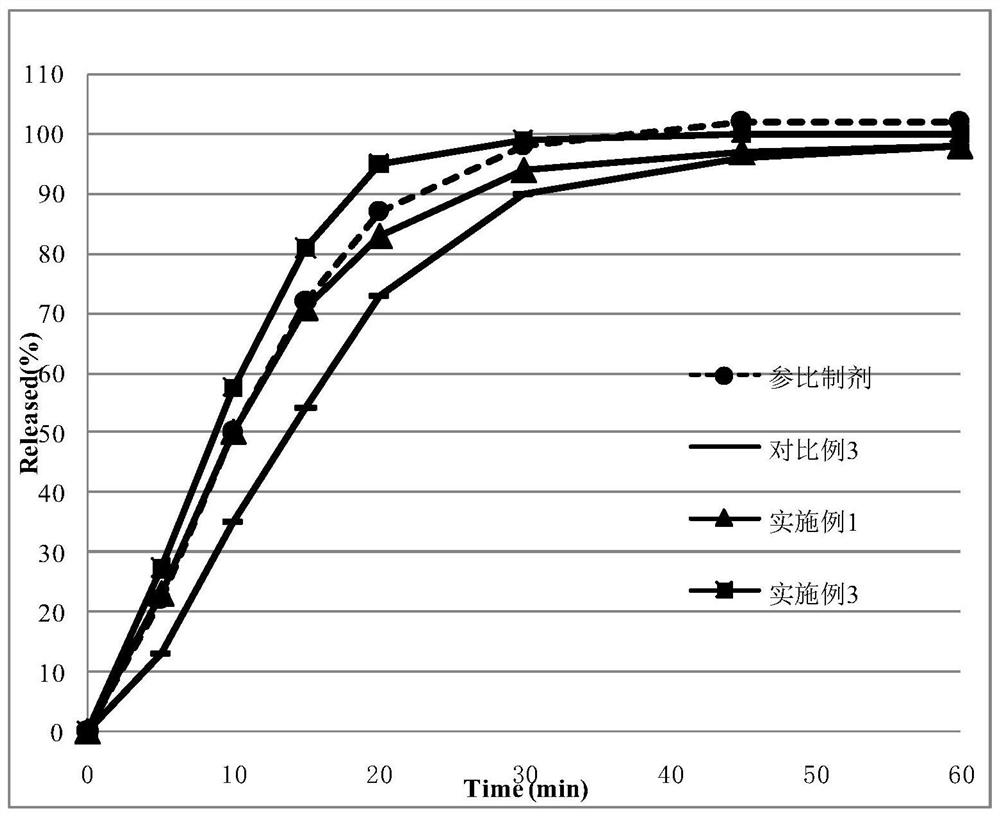
[0136] image 3 Dissolution curves of the tablets prepared for Example 1, Example 3 and Comparative Example 3 and the original drug of the reference preparation in pH2.0 hydrochloric acid. Depend on image 3 It can be seen that after the disintegrants of Example 1 and Example 3 of the present invention adopt the mode of internal and external addition, the dissolution curve is similar to that of the reference preparation (Example 1: f 2 =78; Embodiment 3: f 2 =60. when f 2 When ≥50, it shows that the two preparations are similar, otherwise they are not similar), and the dissolution curve is slower than the reference preparation after the disintegrant is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com