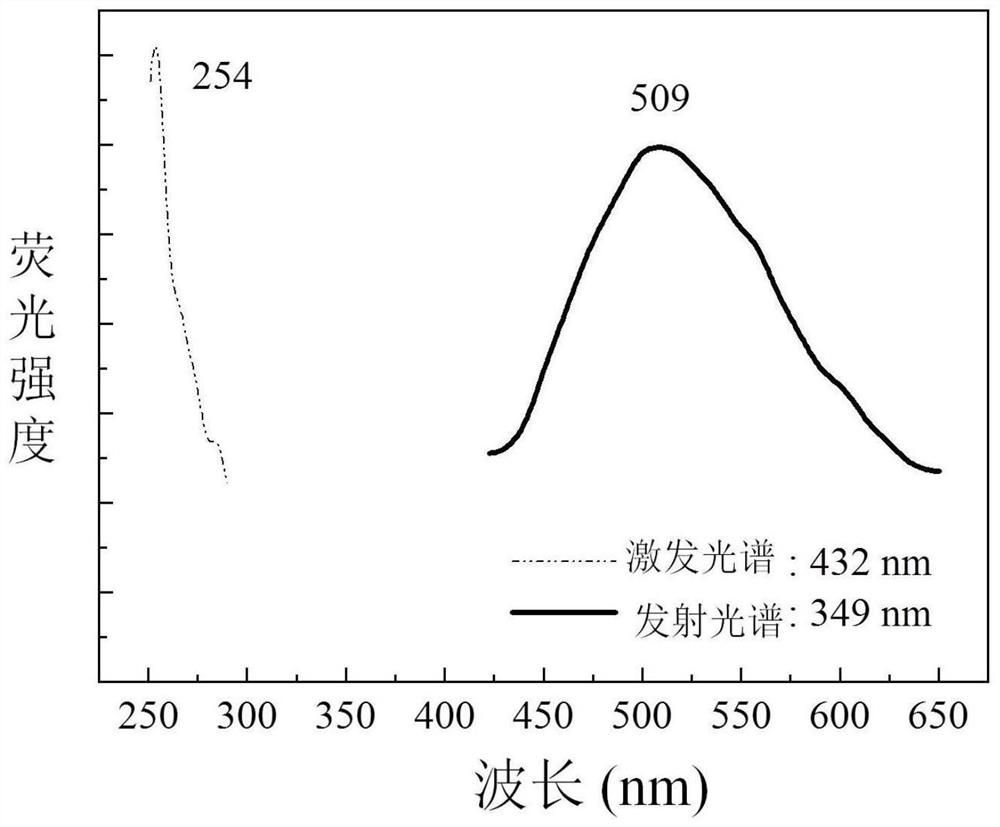
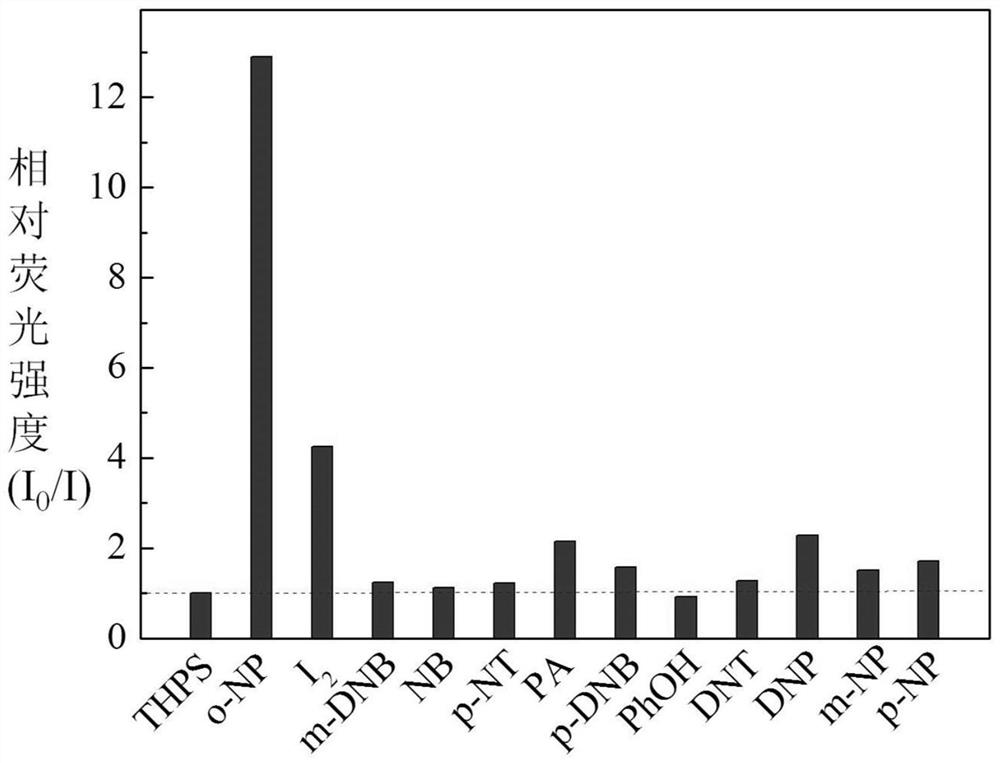
Phenylsilole-based conjugated microporous polymer as well as preparation method and application thereof
A phenylsilole-based and conjugated microporous technology, which is applied in the field of fluorescence sensing and detection, can solve problems such as health risks, no reports of phenylsilole-based conjugated microporous polymers, etc., and achieves easy operation, Good chemical and thermal stability, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of phenylsilolyl conjugated microporous polymer, comprising the steps of:
[0031] Hexaphenylsilole (0.8081g, 1.5mmol), cyanuric chloride (0.5532g, 3mmol), anhydrous AlCl 3 (1.4401g, 10.8mmol) was added to anhydrous dichloromethane (30mL), stirred evenly at room temperature; and nitrogen was passed through, placed in an oil bath and heated to 60°C, reacted for 24h, then cooled to room temperature, pumped filtered, the solid product was washed 3 times with methanol and chloroform, and then extracted with methanol and chloroform in a Soxhlet extractor for 24 hours, and finally dried in a vacuum oven at 50°C to obtain phenylsilolyl conjugated microporous polymer Things, denoted as THPS.
Embodiment 2
[0033] A preparation method of phenylsilolyl conjugated microporous polymer, comprising the steps of:
[0034] 1,1-dimethyl-2,3,4,5-tetraphenylsilole (0.6219g, 1.5mmol), cyanuric chloride (0.3690g, 2mmol), anhydrous AlCl 3(1.4401g, 10.8mmol) was added to chloroform (30mL), stirred evenly at room temperature; and nitrogen was passed through, placed in an oil bath and heated to 80°C, reacted for 20h, then cooled to room temperature, suction filtered, and the solid product Wash with methanol and chloroform three times respectively, then extract with methanol and chloroform in a Soxhlet extractor for 24 hours, and finally dry in a vacuum oven at 60°C to obtain a phenylsilolyl-conjugated microporous polymer, which is denoted as TTPs.
Embodiment 3
[0036] A preparation method of phenylsilolyl conjugated microporous polymer, comprising the steps of:
[0037] 1-methyl-1,2,3,4,5-pentaphenylsilole (0.7150g, 1.5mmol), cyanuric chloride (0.4613g, 2.5mmol), anhydrous AlCl 3 (1.4401g, 10.8mmol) was added to anhydrous dichloroethane (30mL), stirred evenly at room temperature; and nitrogen was passed through, placed in an oil bath and heated to 100°C, reacted for 16h, and then cooled to room temperature, Suction filtration, the solid product was washed 3 times with methanol and chloroform respectively, and then extracted with methanol and chloroform in a Soxhlet extractor for 24 hours, and finally dried in a vacuum oven at 100°C to obtain phenylsilolyl-conjugated microporous Polymer, denoted as TPPS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com