Seven-membered fluorine boron fluorescent dye and application thereof in fluorescent anti-counterfeiting film
A technology of fluorescent dyes and fluorine boron, applied in the field of fluorescent dyes, can solve the problem that the hidden technology cannot be found, and achieve the effects of high solid fluorescence quantum yield, large fluorescence quantum yield and low manufacturing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
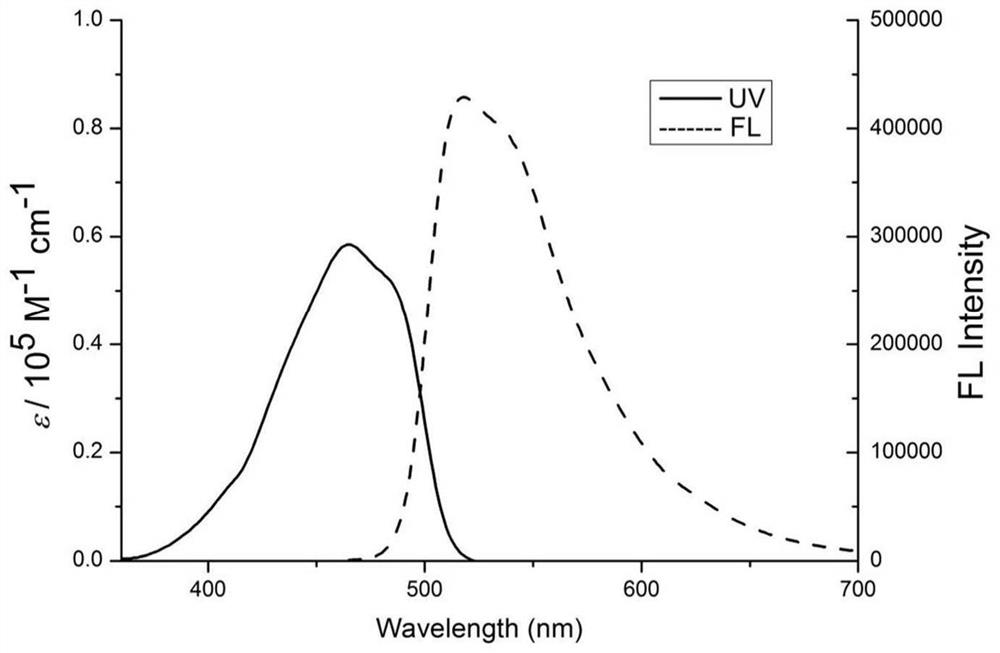
Examples
Embodiment 1
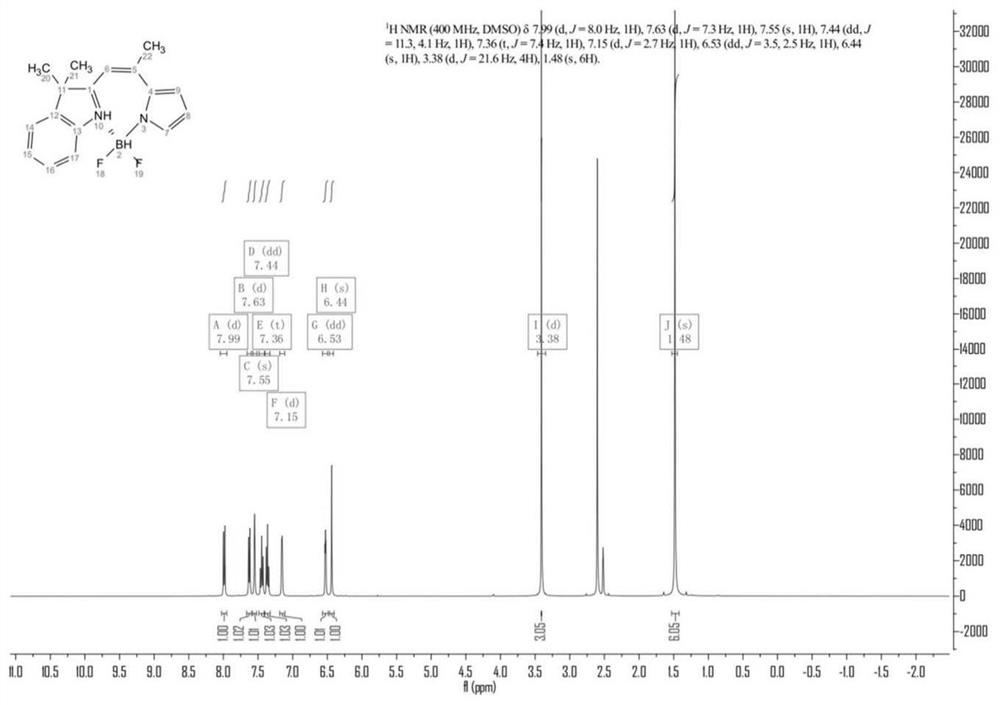
[0026] Weigh 2,3,3-trimethylindole (632mg, 4mmol), take 100.00mL of toluene, mix and dissolve, then add 2-acetylpyrrole (436mg, 4mmol), piperidine (0.36mL, 4mmol), Acetic acid (0.24mL, 4mmol) and phosphorus oxychloride (0.44mL, 4mmol) were heated and stirred at 120°C for 24 hours to complete the reaction. ).
[0027] If the reaction raw material is 2,3,3-trimethylindole, the yield of compound 3 is extremely low, which is not conducive to the collection of compound I. If the yield of compound 3 is high, it is beneficial to the collection of compound 1.
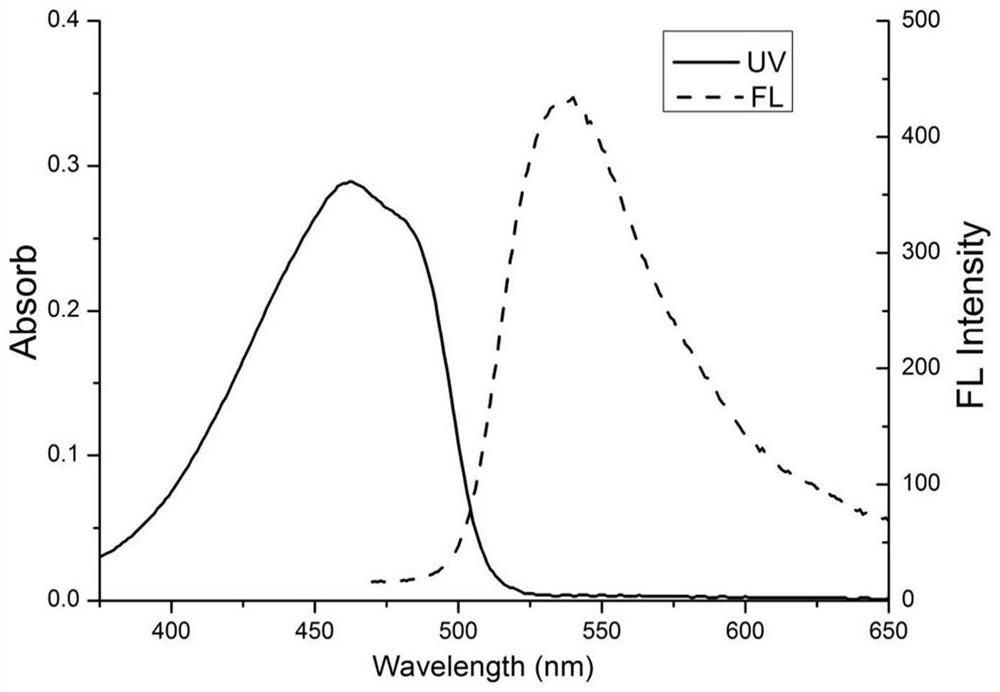
Embodiment 2
[0029] Weigh the protonated 2,3,3-trimethylindole (640mg, 4mmol) of compound 1, take 100.00mL toluene, mix and dissolve, then add 2-acetylpyrrole (436mg, 4mmol), piperidine ( 0.36mL, 4mmol), acetic acid (0.24mL, 4mmol), phosphorus oxychloride (0.44mL, 4mmol) were heated and stirred at 120°C for 24 hours to complete the reaction, and the reactant was rotary evaporated, and purified by column chromatography to obtain light yellow solid 3 ( 120 mg, yield 12%). Take the above compound 3 (120mg, 0.48mmol), take 30ml of toluene solution, 1.00ml of triethylamine, slowly add boron trifluoride ether solution (1.00ml) dropwise under electromagnetic stirring, raise the temperature to 120°C, and reflux After 30 min, TLC plate detection, the product was washed with water, extracted, dried, rotary evaporated, filtered and purified to obtain compound I as a yellow solid (123 mg, yield 86%).
[0030]
Embodiment 3
[0032] Weigh the protonated 2,3,3-trimethylindole (960mg, 6mmol) of compound 1, mix and dissolve 100.00mL toluene, then add 2-acetylpyrrole (436mg, 4mmol) and piperidine ( 0.36mL, 4mmol), acetic acid (0.24mL, 4mmol), phosphorus oxychloride (0.44mL, 4mmol) were heated and stirred at 120°C for 20 hours to complete the reaction, the reactant was rotary evaporated, and purified by column chromatography to obtain a light yellow solid 3 ( 340 mg, yield: 34%). Take the above compound 3 (120mg, 0.48mmol), take 30ml of toluene solution, 1.50ml of triethylamine, slowly add boron trifluoride ether solution (1.50ml) dropwise under electromagnetic stirring, raise the temperature to 120°C, and reflux After 30 min, TLC plate detection, the product was washed with water, extracted, dried, rotary evaporated, filtered and purified to obtain compound I as a yellow solid (132 mg, yield 92%). When the amount of Compound 1 was increased by 1.5 times (relative to Example 1), the yield of Compound 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com