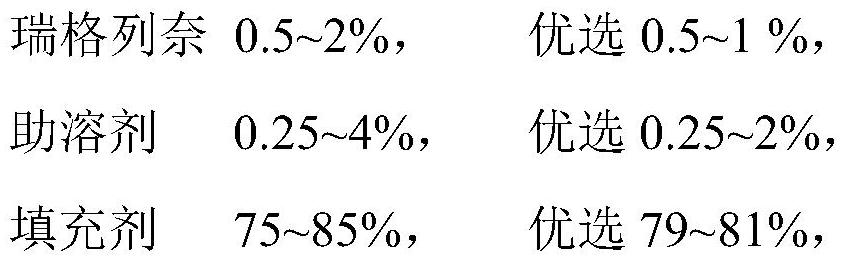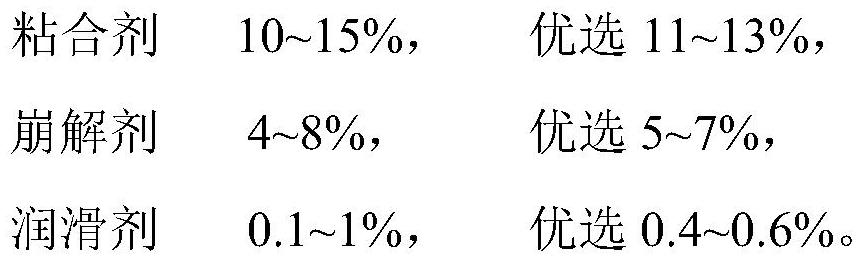Pharmaceutical composition containing repaglinide and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of gastrointestinal mucosal side effects, poor water solubility and dissolution stability of repaglinide, etc., and achieve the effect of improving water solubility, good dissolution stability and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
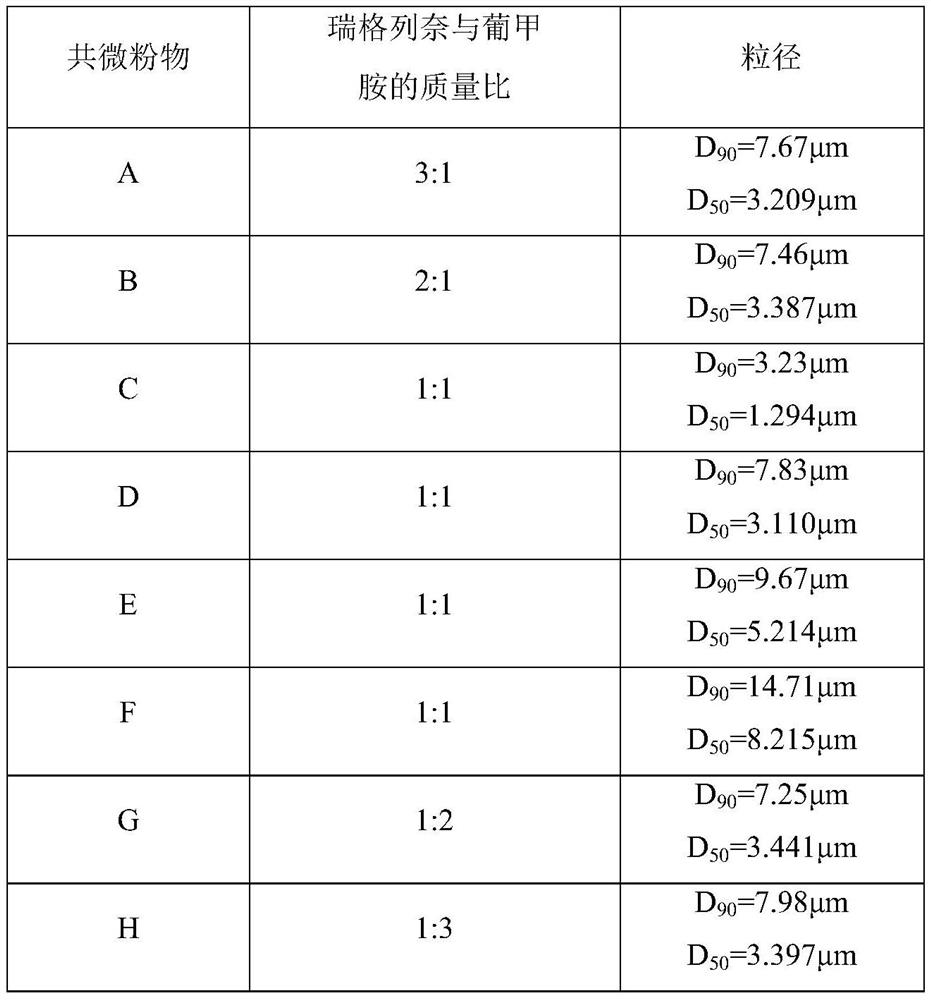
[0033] (1) Preparation of co-micropowder
[0034]
[0035] As shown in the table above, after sieving meglumine, repaglinide was weighed according to the mass ratio and mixed with meglumine, and the micropowder parameters were set for co-micropowder to obtain co-micropowder of different particle sizes.
[0036] (2) Prescription
[0037]
[0038]
[0039] (3) Preparation method
[0040] According to the above table, weigh the co-micron powder and add the auxiliary materials. After mixing, dry granulation. Control the oil pressure of 60-70 bar, the roller speed of 9 rpm, the gap between the rollers of 0.8-1.2 mm, the granulating screen of 0.8 mm, and the speed of granulating. 250 rpm, add the adjuncts, mix well and press into tablets.
Embodiment 9
[0042] The recipe and process of Example 4 were used for scale-up production, with a scale of 100,000 / batch, and three batches were produced in parallel.
experiment example 10
[0066] Experimental Example 10: Dissolution Profile
[0067] According to the dissolution test method (the second method of four general rules of Chinese Pharmacopoeia 2020 edition), add water to 900 mL of pH5.0 citric acid / phosphate buffer (10.2 g of citric acid monohydrate and 18.16 g of disodium hydrogen phosphate dihydrate). Dissolve to 1000mL) as the dissolution medium, set the rotation speed to 75rpm, operate according to the law, take an appropriate amount of the solution at 5min, 10min, 15min and 30min respectively, filter, take the subsequent filtrate as the test solution, according to the import drug registration of repaglinide tablets Standard (JX20120236) chromatographic conditions under the content determination item, set the injection volume to 100 μL, and record the chromatogram; another about 22 mg of repaglinide reference substance, accurately weighed, placed in a 100 mL volumetric flask, dissolved in methanol and diluted to the mark , shake well, accurately m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com